- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
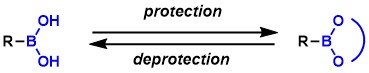
Boronic acids are stable towards air and moisture and are usually solids that can be handled easily. Even though they are undoubtedly an important class of reagents used for the Suzuki-Miyaura coupling and other useful reactions, the use of unprotected boronic acids is not free from drawbacks, including cumbersome purification, complexity of quantitative assay due to trimeric boroxin formation, and instability under acidic and oxidative conditions (for certain boronic acids). To circumvent these problems, boronic acids are often used in protected forms.
-
Examples of Protecting Groups
Commonly used protecting groups are listed below. Many of them are cyclic boronic acid esters. Their stability against hydrolysis increases with the steric hindrance around the boronic acid ester (Conversely, there are cases where boronic acids are used to protect diols). Acyclic counterparts are rarely used for protection purposes.

・Pinacol ester (pin): Pinacol boronate is the most popular protecting group. It can be introduced by the Miyaura borylation or the Hartwig C-H borylation. It is stable enough for column purification but reactive enough to be used directly for the Suzuki coupling. It is so stable that intended hydrolysis or conversion to other boronic acid derivatives is sometimes difficult.
・Diaminonaphthaleneamide (dan): R-B(dan) is very stable under a wide range of conditions. The neighboring nitrogen atoms donate electron density from their lone pair into the empty orbital of the boron, decreasing the Lewis acidity and hence the reactivity.
・MIDA ester: See the separate page for more information. The coordination of the nitrogen atom makes it stable under hydrolytic, oxidative, and reductive conditions (and column purification too). A demerit is the somewhat laborious preparation that requires vigorous removal of water and DMSO.
・Trifluoroborate salt: See the separate page for more information. Trifluoroborate salts tend to have high crystallinity and low solubility in organic solvents. Since the three fluorine atoms donate electron density to fill the empty orbital of the boron, trifluoroborates are highly stable towards oxidation.
・Other examples: Additional examples include catechol ester (cat), neopentylglycol ester (neo), pinanediol ester (highly hydrolysis-resistant), biscyclohexyldiol ester, MPMP ester (oxidatively removable), and cyclic triolborate salt (improved version of trifluoroborate salt).
-
General References
<Reviews>
- Duggan, P. J.; Tyndall, E. M. J. Chem. Soc. Perkin Trans. 1 2002, 1325. DOI: 10.1039/B006767I
- Lennox, A. J. J.; Lloyd-Jones, G. C. Isr. J. Chem. 2010, 50, 664. DOI: 10.1002/ijch.201000074
- Lennox, A. J. J.; Lloyd-Jones, G. C. Chem. Soc. Rev. 2014, 43, 412. DOI: 10.1039/C3CS60197H
- Xu, L.; Zhang, S.; Li, P. Chem. Soc. Rev. 2015, 44, 8848. DOI: 10.1039/C5CS00338E
<diaminonaphthalene>
- Noguchi, H.; Hojo, K.; Suginome, M. J. Am. Chem. Soc. 2007, 129, 758. DOI: 10.1021/ja067975p
<MPMP diol ester>
- Yan, J.; Jin, S.; Wang, B. Tetrahedron Lett. 2005, 46, 8503. doi:10.1016/j.tetlet.2005.10.010
<MIDA boronate>
- Gillis, E. P.; Burke, M. D. J. Am. Chem. Soc. 2007, 129, 6716. DOI: 10.1021/ja0716204
- Li, J.; Grillo, A. S; Burke, M. D. Acc. Chem. Res. 2015, 48, 2297. DOI: 10.1021/acs.accounts.5b00128
<trifluoroborate>
- Vedejs, E.; Chapman, R. W.; Fields, S. C.; Lin, S.; Schrimpf, M. R. J. Org. Chem. 1995, 60, 3020. DOI: 10.1021/jo00115a016
- Darses, S.; Genet, J.-P. Chem. Rev. 2008, 108, 288. DOI: 10.1021/cr0509758
<cyclic triolborate>
- Yamamoto, Y.; Takizawa, M.; Yu, X.-Q,; Miyaura, N. Angew. Chem. Int. Ed. 2008, 47, 928. DOI: 10.1002/anie.200704162
-
Typical Deprotection Conditions
Deprotection of pinacol ester[1]: Direct hydrolysis often requires acidic conditions and sometimes heating. The byproduct pinacol is commonly trapped by NaIO4 or phenylboronic acid. Stepwise deprotection methods via trifluoroborate or aminoborate are milder alternatives.

Deprotection of dan group[2]: After acidic hydrolysis, diaminonaphthalene can be removed easily by extraction.

Deprotection of MIDA ester[3]: MIDA ester can be deprotected easily by basic hydrolysis. It is stable under other conditions.

Interconversion of protecting groups.[4]

-
Experimental Tips
It has been reported that silica gel mixed with boric acid is effective for the purification of pinacol esters.[5]
-
References
- (a, b) Coutts, S. J.; Adams, J.; Krolikowski, D.; Show, R. J. Tetrahedron Lett. 1994, 35, 5109. doi:10.1016/S0040-4039(00)77040-7 (c) Sun, J.; Perfetti, J. S.; Santos, W. L. J. Org. Chem. 2011, 76, 3571. DOI: 10.1021/jo200250y (d) Yuen, A. K. L.; Hutton ,C. A. Tetrahedron Lett. 2005, 46, 7899. doi:10.1016/j.tetlet.2005.09.101
- Noguchi, H.; Hojo, K.; Suginome, M. J. Am. Chem. Soc. 2007, 129, 758. DOI: 10.1021/ja067975p
- Gillis, E. P.; Burke, M. D. J. Am. Chem. Soc. 2007, 129, 6716. DOI: 10.1021/ja0716204
- Churches, Q. I.; Hooper, J. F.; Hutton, C. A. J. Org. Chem. 2015, 80, 5428. DOI: 10.1021/acs.joc.5b00182
- Hitosugi, S.; Tanimoto, D.; Nakanishi, W.; Isobe, H. Chem. Lett. 2012, 41, 972. doi:10.1246/cl.2012.972
-
Related Books
[amazonjs asin=”3527325980″ locale=”US” title=”Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials”]
-
External Links