Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
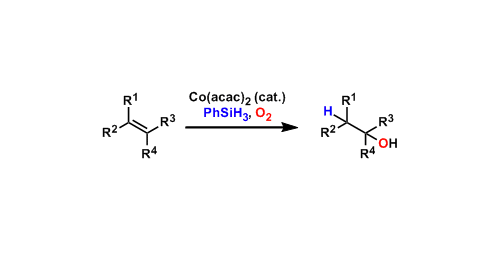
The hydration of alkenes using catalytic cobalt and stoichiometric silane under aerobic conditions was developed by Mukaiyama. The regioselectivity follows the Markovnikov’s rule. Manganese complexes are also known to be effective catalysts.
-
General References
<Stoichiometric examples>
- Mukaiyama, T.; Isayama, S.; Inoki, S.; Kato, K.; Yamada, T.; Takai, T. Chem. Lett. 1989, 449. DOI: 10.1246/cl.1989.449
<Catalytic examples>
- Isayama, S.; Mukaiyama, T. Chem. Lett. 1989, 1071. doi:10.1246/cl.1989.1071
- Inoki, S.; Kato, K.; Takai, T.; Isayama, S.; Yamada, T.; Mukaiyama, T.; Chem. Lett. 1989, 515. DOI: 10.1246/cl.1989.515
- Isayama, S.; Mukaiyama, T. Chem. Lett. 1989, 1071. DOI:10.1246/cl.1989.1071
- Kato, K.; Yamada, T.; Takai, T.; Inoki, S.; Isayama, S. Bull. Chem. Soc. Jpn. 1990, 63, 179 . DOI: 10.1246/bcsj.63.179
<Manganese catalyst (hydration of the α-position of α,β-unsaturated esters)>
- S. Inoki, K. Kato, S.Isayama, T. Mukaiyama, Chem. Lett. 1990, 1869 . DOI: 10.1246/cl.1990.1869
-
Reaction Mechanism
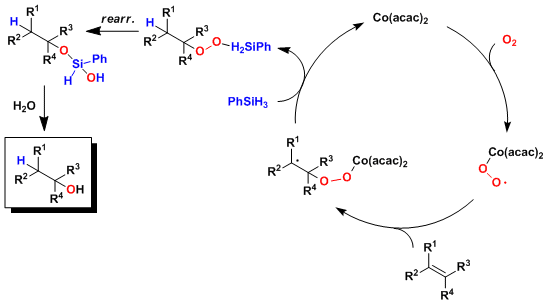
-
Examples
Olefin hydration in the synthesis of cortistatin.[1]
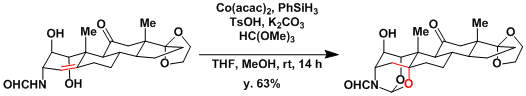
The use of a manganese catalyst in the synthesis of indoxamycin B.[2]

-
References
[1] Shenvi, R. A.; Guerrero, C. A.; Li, C.-C.; Baran, P. S. J. Am. Chem. Soc. 2008, 130, 7241. DOI: 10.1021/ja8023466 [2] Jeker, O.;F, Carreira, E.M. Angew. Chem. Int. Ed. 2012, 51, 3474. DOI: 10.1002/anie.201109175
-
Related Reactions
-
Related Books
-
External Links
・Hydration reaction – Wikipedia