Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
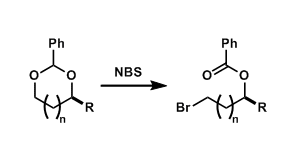
By the treatment with N-bromosuccinimide (NBS), benzylidene acetals can be oxidatively opened. The bromine ends up on the less hindered side of the two hydroxyl groups.
-
General References
- Failla, D. L.; Hullar, T. L.; Siskin, S. B. Chem. Commun. 1966, 716. DOI: 10.1039/C19660000716
- Hanessian, S. Carbohydrate Res. 1966, 2, 86. doi:10.1016/S0008-6215(00)81783-8
-
Reaction Mechanism
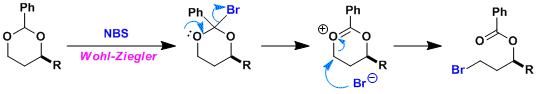
The Wohl-Ziegler bromination is the first step.
-
Examples
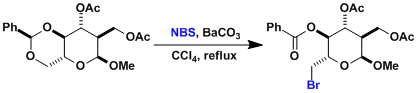
The synthesis of cyclophellitol.[1]
-
References
[1] Sato, K.-i.; Bokura, M.; Moriyama, H.; Igarashi, T. Chem. Lett. 1994, 23, 37. doi:10.1246/cl.1994.37
-
Related Reactions
-
Related Books
-
External Links