Overall Score3.5
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
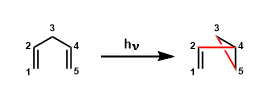
1,4-Dienes undergo light-promoted rearrangement to give vinylcyclopropanes. The scope of this reaction includes dienes containing heteroatoms such as oxygen and nitrogen and even certain benzene ring skeletons.
-
General References
- Zimmerman, H. E.; Binkley, R. W.; Givens, R. S.; Sherwin, M. A. J. Am. Chem. Soc. 1967, 89, 3932. DOI: 10.1021/ja00991a064
- Houk, K. N. Chem. Rev. 1976, 76, 1. DOI: 10.1021/cr60299a001
- Demuth, M.; Schaffner, K. Angew. Chem. Int. Ed. 1982, 21, 820. DOI: 10.1002/anie.198208201
- Zimmerman, H. E.; Armesto, D. Chem. Rev. 1996, 96, 3065. doi:10.1021/cr910109c
<photochemical reactions in total synthesis>
- Hoffmann, N. Chem. Rev. 2008, 108, 1052. DOI: 10.1021/cr0680336
- Bach, T.; Hehn, J. P. Angew. Chem. Int. Ed. 2011, 50, 1000. DOI: 10.1002/anie.201002845
-
Reaction Mechanism
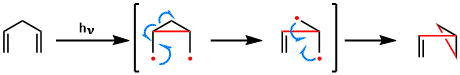
-
Examples
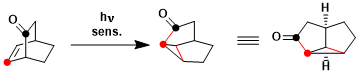
An example of oxa-di-π-methane rearrangement.[1]
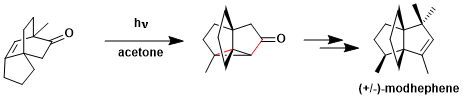
The synthesis of modhephene utilizing oxa-di-π-methane rearrangement.[2]
-
Experimental Tips
-
References
[1] Givens, R. S.; Oettle, W. F.; Coffin, R. L.; Carlson, R. G. J. Am. Chem. Soc. 1971, 93, 3957. DOI: 10.1021/ja00745a024
[2] Mehta, G.; Subrahmanyam, D. J. Chem. Soc. Chem. Commun. 1985, 768. DOI: 10.1039/C39850000768
-
Related Reactions
-
Related Books
[amazonjs asin=”1891389572″ locale=”US” title=”Principles of Molecular Photochemistry: An Introduction”]
-
External Links
- Di-pi-methane rearrangement – Wikipedia
- di-π-methane rearrangement (IUPAC Goldbook)