- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
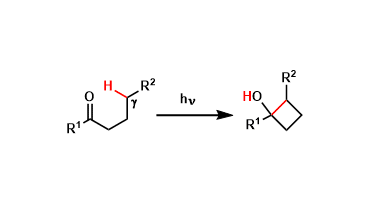
In the Norrish-Yang reaction, the C-H bond at the γ-position of ketones is homolytically cleaved by photo-irradiation and the ensuing rearrangement leads to the formation of cyclobutanols.
-
General References
- Yang, N. C.; Yang, D.-D. H. J. Am. Chem. Soc. 1958, 80, 2913. DOI: 10.1021/ja01544a092
<Photochemical reactions in total synthesis>
- Hoffmann, N. Chem. Rev. 2008, 108, 1052. DOI: 10.1021/cr0680336
- Bach, T.; Hehn, J. P. Angew. Chem. Int. Ed.2011, 50, 1000. DOI: 10.1002/anie.201002845
-
Reaction Mechanism
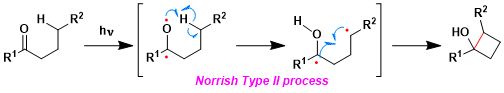
The radicals formed by the Norrish type II reaction form a new C-C bond.
-
Examples
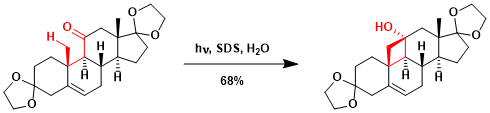
An application to the synthesis of ouabagenin.[1]
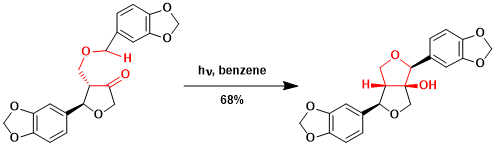
The synthesis of paulownin[2]: This is an interesting variant case where the γ-position does not have a hydrogen.
Ketones containing a leaving group at the α-position undergo different modes of cyclization, such as the example shown here.[3]
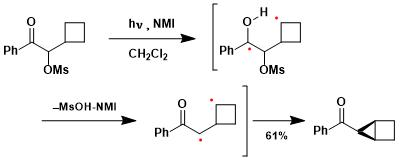
-
Experimental Tips
-
References
[1] Renata, H.; Zhou, Q.; Baran, P. S. Science 2013, 339, 59. doi:10.1126/science.1230631
[2] Kraus, G. A.; Chen, L. J. Am. Chem. Soc. 1990, 112, 3464. DOI: 10.1021/ja00165a033
[3] (a) Wessig, P.; Muhling, O. Angew. Chem. Int. Ed. 2001, 40, 1064. [abstract] (b) Wessig, P.; Muhling, O. Helv. Chim. Acta 2003, 86, 865. DOI: 10.1002/hlca.200390086
-
Related Reactions
-
Related Books
-
External Links