Overall Score3.5
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
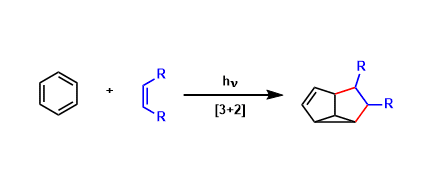
Aromatic rings and alkenes undergo [3+2] cycloaddition under photo-irradiated conditions. This reaction provides complex fused ring skeletons that are otherwise difficult to obtain. Synthetically speaking, site-selective intramolecular versions are especially useful.
-
General References
- Cornelisse, J. Chem. Rev. 1993, 93, 615. DOI: 10.1021/cr00018a002
- Hoffmann, N. Synthesis 2004, 481. DOI: 10.1055/s-2004-815973
- Chappell, D.; Russell, A. T. Org. Biomol. Chem.2006, 4, 4409. DOI: 10.1039/B614011B
- Streit, U.; Bochet, C. G. Beilstein J. Org. Chem. 2011, 7, 525. doi:10.3762/bjoc.7.61
<Photochemical reactions in total synthesis>
- Hoffmann, N. Chem. Rev. 2008, 108, 1052. DOI: 10.1021/cr0680336
- Bach, T.; Hehn, J. P. Angew. Chem. Int. Ed.2011, 50, 1000. DOI: 10.1002/anie.201002845
-
Reaction Mechanism
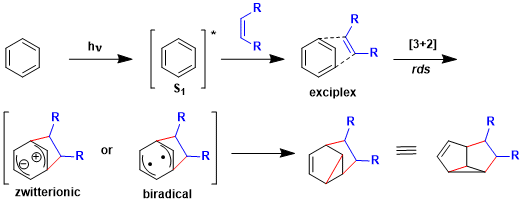
The reaction is considered to proceed via either the zwitterionic or biradical intermediate, with the α bond formation step being rate-determining. Since the product of competitive [2+2] cycloaddition is less stable, [3+2] cycloaddtion predominates.
-
Examples
The synthesis of retigeranic acid.[1]
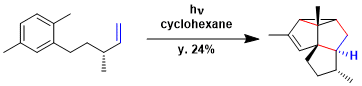
-
Experimental Tips
-
References
[1] Wender, P. A.; Singh, S. K. Tetrahedron Lett. 1990, 31, 2517. doi:10.1016/0040-4039(90)80114-2
-
Related Reactions
-
Related Books
-
External Links