-
General Characteristics
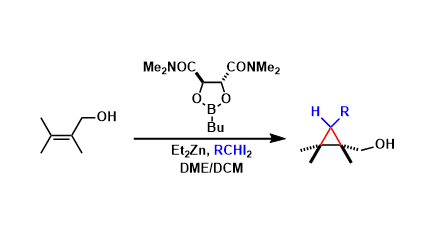
The Simmons-Smith cyclopropanation can be rendered asymmetric by using the chiral boronic acid ester as a reagent. An allylic hydroxyl group is needed in substrates as a directing group.
-
General References
- Charette, A. B.; Juteau, H. J. Am. Chem. Soc. 1994, 116, 2651. DOI: 10.1021/ja00085a068
- Charette, A. B.; Lemay, J. Angew. Chem. Int. Ed.1997, 36, 1090. DOI: 10.1002/anie.199710901
- Charette, A. B.; Juteau, H.; Lebel, H.; Molinaro, C. J. Am. Chem. Soc.1998, 120, 11943. DOI: 10.1021/ja982055v
- Lebel, H.; Marcoux, J.-F.; Molinaro, C.; Charette, A. B. Chem. Rev.2003, 103, 977. doi:10.1021/cr010007e
- Pellissier, H. Tetrahedron2008, 64, 7041. doi:10.1016/j.tet.2008.04.079
-
Reaction Mechanism
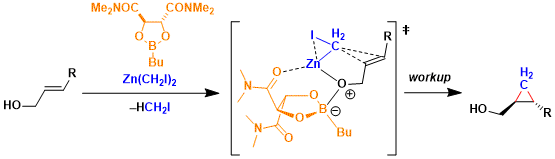
-
Examples
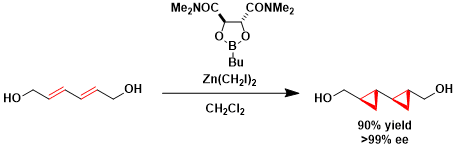
Asymmetric cyclopropanation of a conjugated diene in the synthesis of FR-900848.[1]
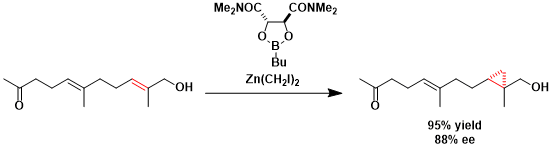
An example in which cyclopropanation is done selectively for the alkene closer to the hydroxyl group.[2]
-
Experimental Tips
-
References
[1] Barrett, A. G. M.; Doubleday, W. W.; Hamprechet, D.; Kasdorf, K.; Tustin, G. J.; White, A. J. P.; Williams, D. J. Chem. Commun. 1997, 1693. DOI: 10.1039/A700487G
[2] (a) Charette, A. B.; Juteau, H.; Lebel, H.; Deschenes, D. Tetrahedron Lett. 1996, 37, 7925. doi:10.1016/0040-4039(96)01798-4 (b) Charette, A. B.; Juteau, H. Tetrahedron 1997, 53, 16277. doi:10.1016/S0040-4020(97)01014-4
-
Related Reactions
-
Related Books
[amazonjs asin=”3527296255″ locale=”US” title=”Metal Carbenes in Organic Synthesis”]
[amazonjs asin=”0080347126″ locale=”US” title=”Cycloaddition Reactions in Organic Synthesis”]
[amazonjs asin=”1118299884″ locale=”US” title=”Methods and Applications of Cycloaddition Reactions in Organic Syntheses”]
-
External Links
- Simmons-Smith Reaction (organic-chemistry.org)
- Shimmons-Smith反応
- Howard Ensign Simmons Jr. BIOGRAPHICAL MEMOIRS
- Simmons-Smith Reaction (Wikipedia)
- Cyclopropane (Wikipedia)
- Synthesis of cyclopropanes (organic-chemistry.org)