Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
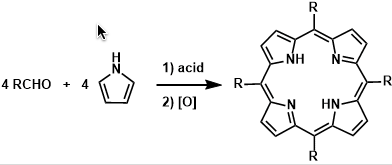
Meso-substituted porphyrins are synthesized by condensation of aldehydes and pyrroles followed by oxidation. The early methods needed forcing conditions but the improvements made by Lindsey allow this reaction to take place at room temperature. High dilution conditions are necessary.
By reacting preformed dipyrromethanes derived from one aldehyde with another kind of aldehyde, it is possible to synthesize C2-symmetric porphyrin derivatives.
-
General References
- Rothemund, P. J. Am. Chem. Soc. 1935, 57, 2010. doi:10.1021/ja01313a510
- Rothemund, P. J. Am. Chem. Soc. 1936, 58, 625. doi:10.1021/ja01295a027
- Adler, A.D.; Longo, F. R.; Shergalis, W. J. Am. Chem. Soc. 1964, 86, 3145. DOI: 10.1021/ja01069a035
- Adler, A. D.; Longo, F. R.; Finarelli, J. D.; Goldmacher, J.; Assour, J.; Korsakoff, L. J. Org. Chem. 1967, 32, 476. doi:10.1021/jo01288a053
- Lindsey, J. S.; Hsu, I. C.; Schreiman, I. C. Tetrahedron Lett.1986, 27, 4969. doi:10.1016/S0040-4039(00)85109-6
- Lindsey, J. S.; Schreiman, I. C.; Hsu, H. C.; Kearney, P. C. J. Org. Chem. 1987, 52, 827. DOI: 10.1021/jo00381a022
- Laha, J. K.; Dhanalekshmi, S.; Taniguchi, M.; Ambroise, A.; Lindsey, J. S. Org. Proc. Res. Dev. 2003, 7, 799. DOI: 10.1021/op034083q
-
Reaction Mechanism
Getting a high yield is usually difficult due to the formation of side products such as pyrrole oligomers.
-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Reactions
-
Related Books
-
External Links