- Generality
- Reagent Availability
- Experimental User Friendliness
- Contribution to Chemical Biology
- Criteria #5
-
General Characteristics
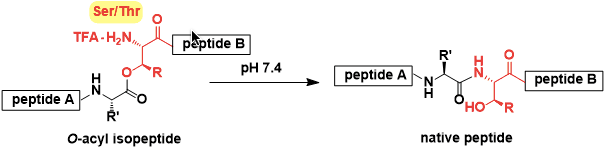
The peptides in which the serine and/or threonine residues are isomerized from amide to ester (with their side chain hydroxyl group) are called O-acylisopeptides.
O-Acylisopeptides show distinct properties from the corresponding native peptides. They can also be converted to the native sequence in aqueous solutions at neutral pH.
The high water solubility of O-acylisopeptides makes them useful in various applications, including the synthesis of difficult hydrophobic sequences, convergent peptide synthesis, and controlled activation of some protein functions.
-
General References
・Sohma, Y.; Sasaki, M.; Hayashi, Y.; Kimura, T.; Kiso, Y. Chem. Commun. 2004, 124. DOI: 10.1039/B312129A
<reviews>
・Sohma, Y.; Yoshiya, T.; Taniguchi, A.; Kimura, T.; Hayashi, T.; Kiso, Y. Pept. Sci. 2007, 88, 253. DOI: 10.1002/bip.20683
・Coin, I.; Beyermann, M.; Bienert, M. Nat. Protoc. 2007, 2, 3247. doi:10.1038/nprot.2007.454
・Sohma, Y.; Kiso, Y. Chem. Rec. 2013, 13, 218. DOI: 10.1002/tcr.201200023
<General Review of Chemical Synthesis of Peptides/Proteins>
・ Humphrey, J. M.; Chamberlin, A. R. Chem. Rev. 1997, 97, 2243. DOI: 10.1021/cr950005s
・ Bray, B. L. Nat. Rev. Drug Discov. 2003, 2, 587. doi:10.1038/nrd1133
・ Nilsson, B. L.; Soellner, M. B.; Raines, R. T. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 91. DOI: 10.1146/annurev.biophys.34.040204.144700・ Kent, S. B. H. Chem. Soc. Rev. 2009, 38, 338. DOI: 10.1039/b700141j
・ Pattabiraman, V. R.; Bode, J. W. Nature 2011, 480, 471. doi:10.1038/nature10702
・ White, C. J.; Yudin, A. K. Nat. Chem. 2011, 3, 509. doi:10.1038/nchem.1062
・ Stolzew, S. C.; Kaiser, M. Synthesis 2012, 44, 1755. DOI: 10.1055/s-0031-1289765
-
Reaction Mechanism

-
Examples
The use of special protecting groups enables the O-to-N rearrangement by external stimuli other than pH. In the example shown below, β-amyloid is converted to the native sequence by photo-irradiation.[1]

-
Experimental Procedure
-
Experimental Tips
- Serine residues are usually used as Boc-protected forms and cleaved/isolated from the solid phase as TFA salts.
- O-Acylated residues can be introduced by solid phase O-acylation under condensation conditions. However, esterification is often less efficient than amidation and racemization can also become problematic. It is usually more reliable to use preformed dipeptide units (Boc-Ser/Thr-(Fmoc-Xaa)-OH) which already contain an ester.
-
References
[1] Kiso, Y. et al. J. Am. Chem. Soc. 2006, 128, 696. DOI: 10.1021/ja057100v
-
Related Reactions
-
Related Books
-
External Links