- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
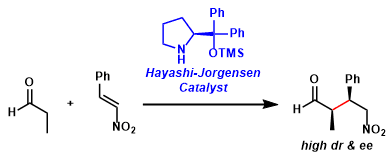
The diarylprolinol silyl ether organocatalysts facilitate various asymmetric reactions of aldehydes by forming active enamine and/or iminium intermediates. These catalysts are generally more active and more soluble in organic solvents than prototypical proline catalyst.
These catalysts were developed independently by Hayashi and Jørgensen around the same time and are referred to as the Hayashi-Jørgensen catalysts. Compared with the MacMillan catalysts that are used often in iminium-mediated reactions, the Hayashi- Jørgensen catalysts tend to be more effective in enamine-mediated reactions.
-
General References
- Marigo, M.; Wabnitz, T. C.; Fielenbach, D.; Jorgensen, K. A. Angew. Chem. Int. Ed. 2005, 44, 794. DOI: 10.1002/anie.200462101
- Hayashi, Y.; Gotoh, H.; Hayashi, T.; Shoji, M. Angew. Chem. Int. Ed. 2005, 44, 4212. DOI: 10.1002/anie.200500599
- Franzen, J.; Marigo, M.; Fielenbach, D.; Wabnitz, T. C.; Kjarsgaard, A.; Jorgensen, K. A. J. Am. Chem. Soc. 2005, 127, 18296. DOI: 10.1021/ja056120u
<reviews>
- Jensen, K. L.; Dickmeiss, G.; Jiang, H.; Albrecht, L.; Jorgensen, K. A. Acc. Chem. Res. 2012, 45, 248. DOI: 10.1021/ar200149w
- Wroblewska, A. Synlett 2012, 23, 953. DOI: 10.1055/s-0031-1290774
-
Reaction Mechanism
The addition of Brønsted acids can have a dramatic influence. Each step of the catalytic cycle is relatively well-understood. (Ref: Helv. Chim. Acta 2011, 94, 719; J. Am. Chem. Soc. 2011, 133, 8822; J. Am. Chem. Soc. 2012, 134, 6741.)

-
Examples
An extremely short synthesis of Tamiflu by Hayashi starting from the organocatalytic asymmetric 1,4-addition.[1]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Ishikawa, H.; Suzuki, T.; Hayashi, Y. Angew. Chem. Int. Ed. 2009, 48, 1304. DOI: 10.1002/anie.200804883
-
Related Reactions
-
Related Books
[amazonjs asin=”3527332367″ locale=”US” title=”Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications, 3 Volume Set”]
[amazonjs asin=”3527305173″ locale=”US” title=”Asymmetric Organocatalysis: From Biomimetic Concepts to Applications in Asymmetric Synthesis”]
[amazonjs asin=”3709111625″ locale=”US” title=”Asymmetric Organocatalysis in Natural Product Syntheses (Progress in the Chemistry of Organic Natural Products)”]
-
External Links
Jorgensen’s Organocatalysis (Sigma-Aldrich)