Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
Zinc borohydride (Zn(BH4)2) is prepared from ZnCl2 and NaBH4. In addition to being used for 1,2-reduction of α,β-unsaturated carbonyl compounds, it can selectively reduce aldehydes in the presence of ketones, and isolated ketones over conjugated ketones.
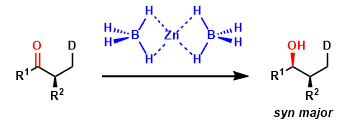
Highly diastereoselective reduction of chelating substrates (such as β-hydroxyketones) is also possible.
-
General References
- Kotsuki, H.; Yoshimura, N.; Ushio, Y.; Ohtsuka, T.; Ochi, M. Chem. Lett. 1986, 1003. doi:10.1246/cl.1986.1003
- Oishi, T.; Nakata, T. Acc. Chem. Res. 1984, 17, 338. DOI: 10.1021/ar00105a007
- Ranu, B. C. Synlett 1993, 885. DOI: 10.1055/s-1993-22640
- Narashimhan, S.; Balakumar, R. Aldrichimica Acta 1998, 31, 19. [PDF]
- Oishi, T.; Nakata, T. e-EROS DOI: 10.1002/047084289X.rz004
- Gama, I. L. Synlett 2012, 23, 642. DOI: 10.1055/s-0031-1290334
-
Reaction Mechanism
The diastereoselectivity in the reduction of chelating substrates can be explained by the general chelation model.
-
Examples

-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Reactions
-
Related Books
-
External Links