- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
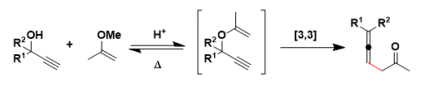
Propargyl vinyl ethers undergo thermal or catalylzed [3,3]-sigmatropic rearrangement to give the corresponding ketoallenes. For chiral compounds, the stereochemistry of the ether is transferred to the axial chirality of the allene. This reaction is also known as the propargyl Claisen rearrangement.
-
General References
・ Saucy, G.; Chopard-dit-Jean, L. H.; Guex, W.; Ryser, G.; Isler, O. Helv. Chim. Acta 1958, 41, 160. DOI: 10.1002/hlca.660410120
・ Marbet, R.; Saucy, G. Chimia 1960, 14, 361.
・ Black, D. K.; Landor, S. R. J. Chem. Soc. 1965,6784. DOI: 10.1039/JR9650006784
・ Saucy, G.; Marbet, R. Helv. Chim. Acta 1967, 50, 1158. DOI: 10.1002/hlca.19670500423
・ Heathcock, C. H.; Henderson, M. A. J. Org. Chem. 1988, 53, 4736. DOI: 10.1021/jo00255a014
・ Tang, Y.; Shen, L.; Dellaria, B. J.; Hsung, R. P. Tetrahedron Lett. 2008, 6404. doi:10.1016/j.tetlet.2008.08.089
・ Cao, T.; Deitch, J.; Linton ,E. C.; Kozlowski, M. C. Angew. Chem. Int. Ed. 2012, 51, 2448. DOI: 10.1002/anie.201107417
-
Reaction Mechanism
-
Examples
The gold catalyst accelerates the rearrangement.[1]

An impressive example from the synthesis of azadirachtin.[2]

The gold-catalyzed Saucy-Marbet rearrangement was used in the total synthesis of indoxamycin B.[3]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Sherry, B. D.; Toste, F. D. J. Am. Chem. Soc. 2004, 126, 15978. DOI: 10.1021/ja044602k
[2] Veitch, G. E.; Beckmann, E.; Burke, B. J.; Boyer, A.; Maslen, S. L.; Ley, S. V. Angew. Chem. Int. Ed. 2007, 46, 7629. DOI:10.1002/anie.200703027
[3] Jeker, O. F.; Carreira, E. M. Angew. Chem. Int. Ed. 2012, 51, 3474. DOI: 10.1002/anie.201109175
-
Related Reactions
-
Related Books
[amazonjs asin=”3527308253″ locale=”US” title=”The Claisen Rearrangement: Methods and Applications”]
-
External Links
・Pericyclic reaction (PDF, Evans Group)
・The Saucy-Marbet reaction (NNNS Blog)
・Indoxamycin B (Chemistry World)