- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
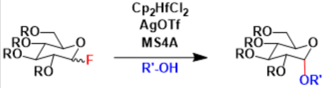
The use of fluorinated sugars as glycosyl donors in glycosylation reactions was first reported by Mukaiyama. The original method employed a cationic tin-based Lewis acid as an activator, and later Suzuki reported more reactive and widely applicable systems employing cationic zirconocene and hafnocene complexes (the Suzuki modification).
Glycosyl fluorides can be activated by other reagents too, including LiClO4 and strong Brønsted acids like TfOH.
-
General References
- Mukaiyama, T.; Murai, Y.; Shoda, S. Chem. Lett. 1981, 431. doi:10.1246/cl.1981.431
- Matsumoto, T.; Maeta, H.; Suzuki, K.; Tsuchihashi, G. Tetrahedron Lett. 1988, 29, 3567. doi:10.1016/0040-4039(88)85294-8
- Suzuki, K.; Maeta, H.; Matsumoto, T.; Tsuchihashi, G. Tetrahedron Lett. 1988, 29, 3571. doi:10.1016/0040-4039(88)85295-X
- Suzuki, K.; Maeta, H.; Matsumoto, T. Tetrahedron Lett. 1989, 30, 4853. doi:10.1016/S0040-4039(01)80526-8
<reviews>
- 鈴木啓介、長澤徹哉 有機合成化学協会誌 1992, 50, 378. doi:10.5059/yukigoseikyokaishi.50.378
- Toshima, K.; Tatsuta, K. Chem. Rev. 1993, 93, 1503. DOI: 10.1021/cr00020a006
- Mukaiyama, T. Tetrahedron 1999, 55, 8609. doi:10.1016/S0040-4020(99)00437-8
- Mukaiyama, T. Angew. Chem. Int. Ed. 2004, 43, 5590. DOI: 10.1002/anie.200300641
- Zhu, X.; Schmidt, R. R. Angew. Chem. Int. Ed. 2009, 48, 1900. DOI: 10.1002/anie.200802036
-
Reaction Mechanism
Glycosyl fluorides are thermally and chemically more stable than other glycosyl halides thanks to the strong carbon-fluorine bond (C-F: 552 kJ/mol, C-Cl: 397 kJ/mol, C-Br: 280 kJ/mol). Glycosyl fluorides are stable enough to be purified by silica gel chromatography.
-
Examples
Total synthesis of benanomicin B.[1]

Total synthesis of gilvocarcin M[2]: Using phenol as an glycosyl acceptor, the O-glycoside rearranges to the C-glycoside at a high temperature.

-
Experimental Procedure
-
Experimental Tips
AgClO4 is very reactive but is potentially dangerous due to the risk of explosion. Trying AgOTf first is recommended.
-
References
- (a) Ohmori, K.; Tamiya, M.; Kitamura, M.; Kato, H.; Oorui, M.; Suzuki, K. Angew. Chem. Int. Ed. 2005, 44, 3871. DOI: 10.1002/anie.200501210 (b) Tamiya, M.; Ohmori, K.; Kitamura, M.; Kato, H.; Arai, T.; Oorui, M.; Suzuki, K. Chem. Eur. J. 2007, 13, 9791. DOI: 10.1002/chem.200700863
- (a) Matsumoto, T.; Hosoya, T.; Suzuki, K. J. Am. Chem. Soc. 1992, 114, 3568. DOI: 10.1021/ja00035a069 (b) Hosoya, T.; Takashiro, E.; Matsumoto, T.; Suzuki, K. J. Am. Chem. Soc. 1994, 116, 1004. DOI: 10.1021/ja00082a023
-
Related Reactions
-
Related Books
[amazonjs asin=”3527317805″ locale=”US” title=”Handbook of Chemical Glycosylation: Advances in Stereoselectivity and Therapeutic Relevance”]
[amazonjs asin=”3642209130″ locale=”US” title=”Reactivity Tuning in Oligosaccharide Assembly (Topics in Current Chemistry)”]
-
External Links