-
General Characteristics
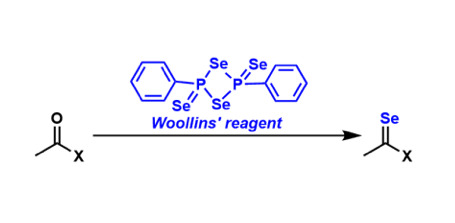
2,4-Diphenyl-1,3-diselenadiphosphetane-2,4-diselenide is called the Woollins’ reagent. In a similar way that the Lawesson’s reagent is used to convert carbonyl groups to the corresponding thiocarbonyl groups, the Woollins’ reagent is used to prepare selenocarbonyl compounds.
-
General References
- Woollins, J. D. et al. J. Chem. Soc., Chem. Commun. 1988, 741.DOI: 10.1039/C39880000741
- Bhattacharyya, P.; Woolins, J. D. Tetrahedron Lett. 2001, 42, 5849. doi:10.1016/S0040-4039(01)01113-3
- Gray, I. P.; Bhattacharyya, P.; Slawin, A. M. Z.; Woollins, J. D. Chem. Eur. J. 2005, 11, 6221. DOI: 10.1002/chem.200500291
- Hua, G.; Li, Y.; Slawin, A. M. Z.; Woolins, J. D. Org. Lett. 2006, 8, 5251. DOI: 10.1021/ol062053c
- Hua, G.; Woolins, J. D. Angew. Chem. Int. Ed. 2009, 48, 1368. doi:10.1002/anie.200800572
<reviews>
- Foreman, M. St. J.; Woollins, J. D. J. Chem. Soc., Dalton Trans. 2000, 1533. doi:10.1016/S0040-4020(01)96753-5
- Lopez-Garcia, M. A. Synlett 2009, 14, 2373. DOI: 10.1055/s-0029-1217802
- Woolins, J. D. Synlett 2012, 1154. DOI: 10.1055/s-0031-1290665
-
Reaction Mechanism
See the page for the Lawesson’s reagent.
-
Examples
The synthesis of selenoamides.[1]

-
Experimental Procedure
-
Experimental Tips
-
References
Bethke, J.; Karaghiosoff, K.; Wessjohann, L. A. Tetrahedron Lett. 2003, 44, 6911. doi:10.1016/S0040-4039(03)01690-3
-
Related Reactions
ローソン試薬 (Lawesson’s reagent)
-
Related Books
-
External Links