Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
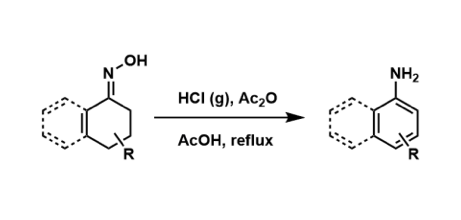
The synthesis of anilines from the corresponding cyclohexenone oximes is known as the Semmler-Wolff reaction. Due to the competing Beckmann rearrangement, yields tend to be low under classical conditions.
-
General References
- Semmler, W. Ber. 1892, 25, 3352.
- Wolff, L. Liebigs Ann. Chem. 1902, 322, 351.
-
Reaction Mechanism

-
Examples
A modified mothod using a palladium catalyst was developed to improve yields.[1] 
The synthesis of meta-substituted anilines.[2] 
An application to the synthesis of penitrem D.[3] 
-
Experimental Procedure
-
Experimental Tips
-
References
- Hong, W. P.; Iosub, A. V.; Stahl, S. S. J. Am. Chem. Soc. 2013, 135, 13664. doi:10.1021/ja4073172
- Kelly, T. R.; Chandrakumar, N. S.; Saha, J. K. J. Org. Chem. 1989, 54, 980. DOI: 10.1021/jo00265a049
- Smith, A. B., III; Kanoh, N.; Ishiyama, H.; Minakawa, N.; Rainier, J. D.; Hartz, R. A.; Cho, Y. S.; Cui, H.; Moser, W. H. J. Am. Chem. Soc. 2003, 125, 8228. DOI: 10.1021/ja034842k
-
Related Reactions
ベックマン転位 (Beckmann Rearrangement)
-
Related Books
-
External Links
- Semmler-Wolff reaction (SynArchive)
- Semmler-Wolff reaction – Wikipedia