- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
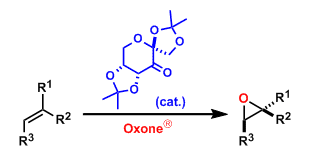
The asymmetric epoxidation of alkenes using the in situ generated chiral dioxirane formed from the fructose-derived ketone pre-catalyst and Oxone® (potassium peroxymonosulfate) is called the Shi epoxidation. The reaction proceeds under basic conditions and within a mild temperature range (0℃~rt). It can be used on a large scale, although the catalyst loading is relatively high (20-30 mol%).
-
General References
- Tu, Y.; Wang, Z.-X.; Shi, Y. J. Am. Chem. Soc. 1996, 118, 9806. DOI: 10.1021/ja962345g
- Wang, Z.-X.; Tu, W; Frohn, M.; Zhang, J.-R.; Shi, Y. J. Am. Chem. Soc. 1997, 119, 11224.doi:10.1021/ja972272g
- Frohn, M.; Shi, Y. Synthesis 2000, 14, 1979. DOI: 10.1055/s-2000-8715
- Adam, W.; Saha-Moeller, C. R.; Zhao, C. -G. Org. React. 2002, 61, 219.
- Shi, Y. Acc. Chem. Res. 2004, 37, 488. DOI: 10.1021/ar030063x
-
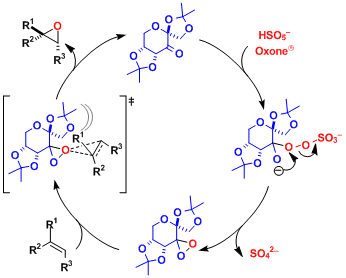
Reaction Mechanism
The in situ generated chiral dioxirane works as the active epoxidation agent. It is important to run the reaction under basic conditions, because under acidic conditions the catalyst is destroyed via the Baeyer-Villiger oxidation.
-
Examples
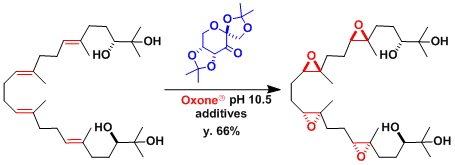
The Shi reaction has been used effectively to epoxidize multiple alkenes, as exemplified in the synthesis of glabrescol.[1]
The polyepoxide prepared by the Shi epoxidation undergoes cascade reaction to give the polyether product.[2]
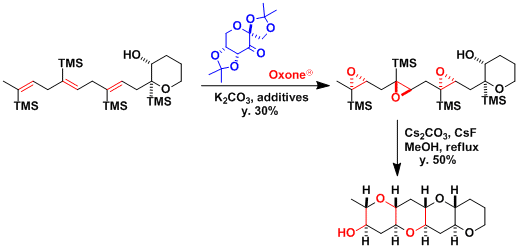
The synthesis of octalactin[3]: When the substrate is a conjugated diene, the more electron-rich and sterically less hindered olefin reacts preferentially.
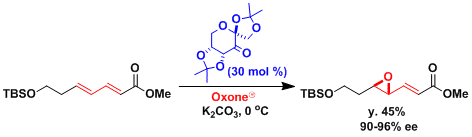
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Xiong, Z.; Corey, E. J. J. Am. Chem. Soc. 2000, 122, 4831, 9328. DOI: 10.1021/ja000842y DOI: 10.1021/ja0024901 [2] Simpson, G. L.; Heffron, T. P.; Merino, E.; Jamison, T. F. J. Am. Chem. Soc. 2006, 128, 1056. DOI: 10.1021/ja057973p [3] Bluet, G.; Campagne, J.-M. Synlett 2000, 221. DOI: 10.1055/s-2000-6491
-
Related Reactions
- dimethyldioxirane
- Rubottom Oxidation
- Jacobsen-Katsuki Epoxidation
- Nucleophilic Epoxidation with Peroxide
- Davis Oxidation
- Prilezhaev Epoxidation
- Sharpless-Katsuki Asymmetric Epoxidation (Sharpless AE)
-
External Links
- Shi Epoxidation (Wikipedia)
- Yian Shi Group (Colorado State University)