- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
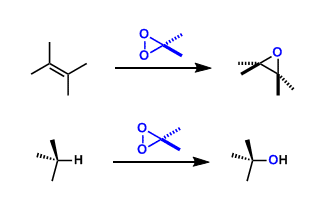
Dimethyldioxirane (DMDO), prepared from acetone and Oxone®, is used frequently to oxidize alkenes to epoxides. Methyl(trifluoromethyl)dioxirane (TFDO) is about 600 times more reactive than DMDO.
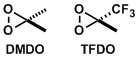
Compared with mCPBA, the advantages of using dioxiranes are neutral reaction conditions, easy workup (since the byproduct is only acetone), and low cost of the reagents. However, dioxirane reagents are unstable and have to be prepared freshly for each experiment. These reagents require purification by vacuum distillation and somewhat special handling.
For certain substrates, direct hydroxylation of unactivated aliphatic C-H bonds is possible.
-
General References
- Murray, R. W.; Jayaraman, R. J. Org. Chem. 1985, 50, 2847. DOI: 10.1021/jo00216a007
- Adam, W.; Chan, Y.-Y.; Cremer, D.; Gauss, J.; Scheutzow, D.; Schindler, M. J. Org. Chem. 1987, 52, 2800. DOI: 10.1021/jo00389a029
- Mello, R.; Fiorentino, M.; Sciacovelli, O.; Curci, R. J. Org. Chem. 1988, 53, 3890. DOI: 10.1021/jo00251a053
- Mello, R.; Fiorentino, M.; Fusco, C.; Curci, R. J. Am. Chem. Soc. 1989, 111, 6749. DOI: 10.1021/ja00199a039
- Adam, W.; Hadjiarapoglou, L.; Nestler, B. Tetrahedron Lett. 1990, 31, 331. doi:10.1016/S0040-4039(00)94547-7
- Yang, D.; Wong, M.-K.; Yip. Y.-C. J. Org. Chem. 1995, 60, 3887. DOI: 10.1021/jo00117a046
- Frohn, M.; Wang, Z.-X.; Shi, Y. J. Org. Chem. 1998, 63, 6425. DOI: 10.1021/jo980604+
- Yang, D.; Wong, M.-K.; Wang, X. -C.; Tang, Y.-C. J. Am. Chem. Soc. 1998, 120, 6611. DOI: 10.1021/ja980916u
- Ferraz, H. M. C.; Muzzi, R. M.; Vieira, T. O.; Viertler, H. Tetrahedron Lett. 2000, 41, 5021. doi:10.1016/S0040-4039(00)00769-3
<reviews>
- Murray, R. W. Chem Rev. 1989, 89, 1187. DOI: 10.1021/cr00095a013
- Curci, R.; Dinoi, A.; Rubino, M. F. Pure Appl. Chem. 1995, 67, 811. doi: 10.1351/pac199567050811
- Curci, R.; D’Accolti, L.; Fusco, C. Acc. Chem. Res. 2006, 39, 1. DOI: 10.1021/ar050163y
- Adam, W.; Saha-Moller, C. R.; Zhao, C.-G. Org. React. 2002, 61, 219.
-
Reaction Mechanism
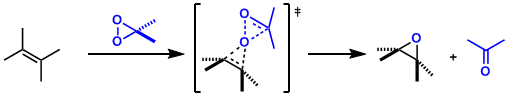
-
Examples
The synthesis of morphine.[1]
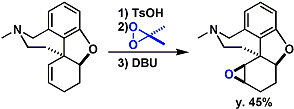
The synthesis of merrilactone.[2]
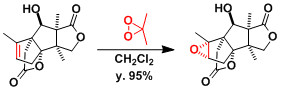
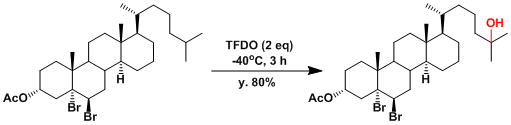
An example of selective C-H oxygenation[3]: Methine carbons are more reactive than methylene carbons.
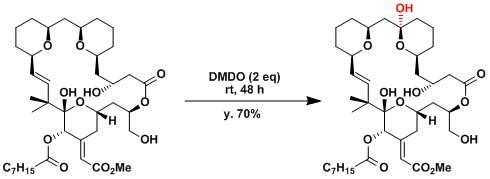
The application to the synthesis of an extremely complex bryostatin analogue.[4]
-
Experimental Procedure
Preparation of dimethyldioxirane.[5a]

ADD EXPERIMENTAL LATER
-
Experimental Tips
*As is always the case with synthesis of peroxide compounds, the preparation of dioxiranes has a risk of explosion. A blast shield should be used.
*DMDO is a volatile peroxide and its handling requires extra caution. Use it only inside a fume hood.
*Touching and inhaling these strong oxidants must be avoided. Appropriate protective gloves should be worn.
-
References
[1] Trost, B. M.; Tang, W.; Toste, F. D. J. Am. Chem. Soc. 2005, 127, 14785. DOI: 10.1021/ja054449+
[2] (a) Inoue, M.; Sato, T.; Hirama, M. J. Am. Chem. Soc. 2003, 125, 10772. DOI: 10.1021/ja036587+ (b) Inoue, M.; Sato, T.; Hirama, M. Angew. Chem. Int. Ed. 2006, 45, 4843. doi:10.1002/anie.200601358 (c) Inoue, M.; Lee, N.; Kasuya, S.; Sato, T.; Hirama, M.; Moriyama, M.; Fukuyama, Y. J. Org. Chem. 2007, 72, 3065. DOI: 10.1021/jo0700474
[3] (a) Bovicelli, P.; Lupattelli, P.; Mincione, E.; Prencipe, T.; Curci, R. J. Org. Chem. 1991, 57, 2182. DOI: 10.1021/jo00033a053 (b) Iida, T.; Yamaguchi, T.; Nakamori, R.; Hikosaka, M.; Mano, N.; Goto, J.; Nambara, T. J. Chem. Soc. Perkin Trans. 1 2001, 2229. DOI: 10.1039/B104938K
[4] Wender, P. A.; Hilinski, M. K.; Mayweb, A, V, W, Org. Lett. 2005, 7, 79. DOI: 10.1021/ol047859w
[5] (a) Li, Y.; Tang, P.; Chen, Y.; Yu, B. J. Org. Chem. 2008, 73, 4323. DOI: 10.1021/jo8003875 (b) Murray, R. W.; Singh, M. Org. Synth. Coll. Vol. 9, 288 (1988). [website]
-
Related Reactions
- Catalytic C-H Oxidation
- Hofmann-Löffler-Freytag Reaction
- Etard Reaction
- Catalytic C-H activation
- Shi Asymmetric Epoxidation
- C-H Insertion of Metal Carbenoid
- Rubottom Oxidation
- Jacobsen-Katsuki Epoxidation
- Davis Oxidation
- Prilezhaev Epoxidation
- Sharpless-Katsuki Asymmetric Epoxidation (Sharpless AE)
-
Related Books
[amazonjs asin=”3527323201″ locale=”US” title=”Modern Oxidation Methods”]
-
External Links