Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
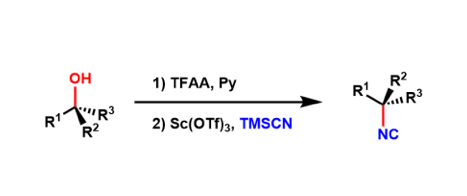
Shenvi reported recently that isonitriles can be prepared by substitution of tertiary alcohols using TMSCN with the inversion of stereochemistry. Isonitriles can be regarded as amine equivalents and are also useful intermediates for multi-component reactions such as the Ugi and the Passerini reactions.
Very interestingly, this reactivity does not apply to primary and secondary alcohols and is limited to tertiary alcohols.
-
General References
Pronin, S. V.; Reiher, C. A.; Shenvi, R. A. Nature 2013, 501, 195. doi:10.1038/nature12472
-
Reaction Mechanism

-
Examples
An application in the synthesis of amphilectene.[1] 
Another example in the context of kalihinol B synthesis.[2]

-
Experimental Procedure
-
Experimental Tips
-
References
- Pronin, S. V.; Shenvi, R. A. J. Am. Chem. Soc. 2012, 134, 19604. DOI: 10.1021/ja310129b
- Daub, M. E.; Prudhomme, J.; Le Roch, K.; Vanderwal, C. D. J. Am. Chem. Soc. 2015, 137, 4912. DOI: 10.1021/jacs.5b01152
-
Related Reactions
-
Related Books
-
External Links
Ryan Shenvi Lab (The Scripps Research Institute)