Overall Score4.5
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
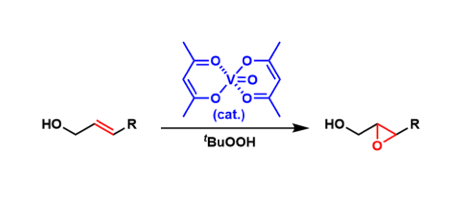
Vanadyl acetylacetonate (VO(acac)2) is used as a catalyst for epoxidation of allylic alcohols (with the use of tert-butyl hydroperoxide as the re-oxidant).
The directing effect of the hydroxyl group within the substrate allows for highly diastereoselective epoxidation of olefins.
-
General References
- Indictor, I.; Brill, W. F. J. Org. Chem. 1965, 30, 2074. DOI: 10.1021/jo01017a520
- Sharpless, K. B.; Michaelson, R. C. J. Am. Chem. Soc. 1973, 95, 6136. DOI: 10.1021/ja00799a061
- Tanaka,S.; Yamamoto, H.; Nozaki, H.; Sharpless, K. B.; Michaelson, R. C.; Cutting, J. D. J. Am. Chem. Soc. 1974, 96, 5254. DOI: 10.1021/ja00823a042
- Chong, A. O.; Sharpless, K. B. J. Org. Chem. 1977, 42, 1587. DOI: 10.1021/jo00429a024
- Michaelson, R. C.; Palermo, R. E.; Sharpless, K. B. J. Am. Chem. Soc. 1977, 99, 1990. DOI: 10.1021/ja00448a059
- Itoh, T.; Jitsukawa, K.; Kaneda, K.; Teranishi, S. J. Am. Chem. Soc. 1979, 101, 159. DOI: 10.1021/ja00495a027
<reviews>
- Sharpless, K. B.; Verhoeven, T. R. Aldrichimica Acta 1979, 12, 63.
- Butler, A.; Clagur, M.; Meister, G. E. Chem. Rev. 1994, 94, 625. DOI: 10.1021/cr00027a004
- Hirao, T. Chem. Rev. 1997, 97, 2707. DOI: 10.1021/cr960014g
<selectivity>
- Hoveyda, A. H.; Evans, D. A.; Fu, G. C. Chem. Rev. 1993, 93, 1307. DOI: 10.1021/cr00020a002
-
Reaction Mechanism

The order of reactivity is: allylic alcohols > homoallylic alcohols > simple alkenes.
-
Examples
An application in the total synthesis of pectenotoxins.[1] 
Selective epoxidation during the synthesis of anisatin.[2] 
Synthesis of fumagillol[3]: The epoxidation of homoallylic alcohols is also possible for certain substrates meeting geometrical requirements.

-
Experimental Procedure
-
Experimental Tips
-
References
- Evans, D. A.; Rajapakse, H. A.; Stenkamp, D. Angew. Chem. Int. Ed. 2002, 41, 4569. [abstract]
- Ogura, A.; Yamada, K.; Yokoshima, S.; Fukuyama, T. Org. Lett. 2012, 14, 1632. DOI: 10.1021/ol300390k
- (a) Vosburg, D. A.; Weiler, S.; Sorensen, E. J. Angew. Chem. Int. Ed. 1999, 38, 971. [abstract] (b) Vosburg, D. A.; Weiler, S.; Sorensen, E. J. Chirality 2003, 15, 156. DOI: 10.1002/chir.10181
-
Related Reactions
- 香月・シャープレスエポキシ化 (Katsuki-Sharpless Epoxidation)
- ジェイコブセン・香月エポキシ化 (Jacobsen-Katsuki Epoxidation)
- プリリツェフエポキシ化 (Prilezhaev Epoxidation)
-
Related Books
-
External Links