- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
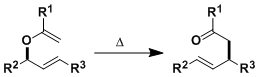
The Claisen rearrangement is the [3,3]-sigmatropic rearrangement of allyl vinyl ethers producing γ,δ-unsaturated carbonyl compounds.
This reactions is analogous to the Cope rearrangement, with the difference being the replacement of a carbon with an oxygen. Whereas the Cope rearrangement is inherently reversible, the Claisen rearrangement is essentially irreversible since the products are substantially more stable than the reactants.
The stereochemistry at the rearranging carbon is transferred to the products (i.e. the reaction occurs stereospecifically). Chiral allyl vinyl ethers can be prepared from chiral ally alcohols, which in turn are accessible via such reactions as the Sharpless epoxidation and the CBS reduction. The rearrangement of chiral substrates is an efficient way to construct synthetically challenging quaternary stereogenic carbon centers and remote stereocenters.
The π bonds of aromatic rings are known to take part in the rearrangement, which is often utilized in the synthesis of substituted phenols.
-
General References
・Claisen, L. Ber. 1912, 45, 3157.
・Claisen, L.; Eisleb, O. Ann. 1914, 401, 21.
・Claisen, L.; Tietze, E. Ber. 1925, 58, 275.
・Claisen, L.; Tietze, E. Ber. 1926, 59, 2344.
・Tarbell, D. S. Org. React. 1944, 2, 1.
・Rhoads, S. A.; Raulins, N. A. Org. React. 1975, 22, 1.
・Ziegler, F. E.Chem. Rev. 1988, 88, 1423. DOI: 10.1021/cr00090a001
・Wipf, P. Comprehensive Organic Synthesis 1991, 5, 827.
・Castro, A. M. M. Chem. Rev. 2004, 104, 2939. DOI: 10.1021/cr020703u
-
Reaction Mechanism
The reaction is a thermally driven [3,3]-sigmatropic rearrangement with concerted bond cleavage and formation. The transition state is considered to take the six membered chair conformation minimizing 1,3-diaxial repulsions, which generally explains the stereoselectivity.
-
Examples
The addition of oxophilic Lewis acids allows the reaction to take place at low temperatures (under kinetically controlled conditions). Using bulky ligands, thermally unstable Z-alkenes can be the major product.[1]
Gold complexes have been shown to work as catalysts with unique activity. The rearrangement of propargyl vinyl ethers catalyzed by Au(I) has been reported.[2]
The biomimetic total synthesis of 1-O-methylforbesione.[3]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Nonoshita, K.; Banno, H.; Maruoka, K.; Yamamoto, H. J. Am. Chem. Soc. 1990, 112. 316. DOI: 10.1021/ja00157a049
[2] Sherry, B. D.; Toste, F. D. J. Am. Chem. Soc. 2004, 126, 15978. DOI: 10.1021/ja044602k
[3] Nicolaou, K. C.; Li, J. Angew. Chem. Int. Ed. 2001, 40, 4264. [abstract]
-
Related Books
[amazonjs asin=”0198503075″ locale=”US” title=”Pericyclic Reactions (Oxford Chemistry Primers)”]

![claisen_2[1]](https://assets.en.chem-station.com/uploads/2014/08/claisen_21.gif)
![Claise2[1]](https://assets.en.chem-station.com/uploads/2014/08/Claise21.gif)
![claisen_4[1]](https://assets.en.chem-station.com/uploads/2014/08/claisen_41.gif)
![claisen_5[1]](https://assets.en.chem-station.com/uploads/2014/08/claisen_51.gif)
![claisen_6[1]](https://assets.en.chem-station.com/uploads/2014/08/claisen_61.gif)