- Generality
- Reagent Availability
- Experimental User Friendliness
- Industrial Importance
- Criteria #5
-
General Characteristics
The Wacker oxidation was originally developed as the process of producing acetaldehyde from ethylene using the PdCl2-CuCl2 cocatalytic system. Molecular oxygen can be used as the terminal oxidant.
The reaction can be used to oxidize various terminal alkenes to give the corresponding methyl ketones. DMF is used often as the solvent.
Internal alkenes are usually unreactive, thus terminal alkenes can be oxidized selectively when both are present in the same molecule.
-
General References
・Smidt, J.; Sieber, R. Angew. Chem. 1959, 1, 176. doi:10.1002/ange.19590710503
・Smidt, J.; Hafner, W.; Jira, R.; Sieber, R.; Sedlmeier, J.; Sabel, A. Angew. Chem. Int. Ed. Engl. 1962, 1, 80. doi:10.1002/anie.196200801
・Tsuji, J. Synthesis 1984, 369.
・辻二郎ら、有機合成化学協会誌 1989, 47, 649.
・Hegedus, L. S. Comprehensive Organic Synthesis, 1991, 4, 552.
・Tsuji, J. Comprehensive Organic Synthesis 1991, 7, 449.
-
Reaction Mechanism
The cooperative catalytic cycle involves the oxidation of the alkene by Pd(II), the oxidation of Pd(0) by Cu(II), and the oxidation of Cu(I) by molecular oxygen.
-
Examples
Terminal alkenes are oxidized more easily than internal alkenes and aldehydes.[1]
A highly stereoselective cyclization reaction by the tandem Wacker-Mizoroki-Heck strategy.[2]
-
Experimental Procedure
The oxidation of 1-decane.[3]
-
Experimental Tips
-
References
[1] Tsuji, J. Synthesis 1984, 369.
[2] Larock, R. C. et al. J. Am. Chem. Soc. 1991, 113, 7815. DOI: 10.1021/ja00020a083
[3] Org. Synth. 1989, 62, 9.
-
Related Books

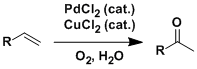
![wacker_oxi_2[1]](https://assets.en.chem-station.com/uploads/2014/07/wacker_oxi_21.gif)
![wacker_oxi_4[1]](https://assets.en.chem-station.com/uploads/2014/07/wacker_oxi_41.gif)
![wacker_oxi_5[1]](https://assets.en.chem-station.com/uploads/2014/07/wacker_oxi_51.gif)
![wacker_oxi_6[1]](https://assets.en.chem-station.com/uploads/2014/07/wacker_oxi_61.gif)