- Generality
- Reagent Availability
- Experimental User Friendliness
- Historical Significance
- Criteria #5
-
General Characteristics
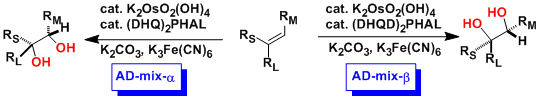
-Sharpless developed chiral ligands ((DHQD)2PHAL or (DHQ)2PHAL) derived from cinchona alkaloids (quinidine, quinine) for the practical osmium tetroxide-catalyzed asymmetric dihydroxylation reaction.

-While NMO is also a viable reagent as the reoxidant, the use of potassium ferricyanide (K3Fe(CN)6) in aqueous systems is particularly effective in achieving high enantioselectivity.
-In general, substrates containing aryl groups tend to give higher reactivity and selectivity (due likely to the π-π interactions in the transition state, as shown in the mechanism section below).
-Additives such as methanesulfonamide (MsNH2) are known to improve enantioselectivity.
-Catalyst/reagent kits are commercially available with the names AD-mix-α and AD-mix-β. The selectivity of each kit follows the empirical rules. 
-There are also “Super-AD-mix” kits introduced by Nicolaou, which contain increased amounts of ligands to boost the reactivity. 
K. Barry Sharpless (Scripps) won the Noble Prize in Chemistry in 2001 for the development of catalytic asymmetric oxidation reactions such as this dihydroxylation and the Sharpless-Katsuki epoxidation.
-
General References
・Hentges, S. G.; Sharpless, K. B. J. Am. Chem. Soc. 1980, 102, 4263. DOI: 10.1021/ja00532a050
・Jacobsen, E. N.; Marko, I.; Mungall, W. S.; Schroder, G.; Sharpless, K. B. J. Am. Chem. Soc. 1988, 110, 1968. DOI: 10.1021/ja00214a053
・Morikawa, K.; Park, J.; Andersson, P. G.; Hashiyama, T.; Sharpless, K. B. J. Am. Chem. Soc. 1993, 115, 8463. DOI: 10.1021/ja00071a072
・Kolb, H.; VanNiewenhze, M. S.; Sharpless, K. B. Chem. Rev. 1994, 94, 2483. DOI: 10.1021/cr00032a009
・Cha, J. K.; Kim, N.-S. Chem. Rev. 1995, 95, 1761. DOI: 10.1021/cr00038a003
・Johnson, R. A.; Sharpless, K. B. Catalytic Asymmetric Synthesis (2nd ed.), 2000, 357.
・Bolm, C.; Hildebrand, J. P.; Muniz, K. Catalytic Asymmetric Synthesis (2nd ed.), 2000, 399.
・Zaitsev, A. B.; Adolfsson, H. Synthesis 2006, 11, 1725. DOI: 10.1055/s-2006-942378
-
Reaction Mechanism
When the dissociation of the diol (the hydrolysis step) is slow, the reaction enters the second cycle, leading to lower enantioselectivity. In terms of preventing it, using aqueous systems and potassium ferricyanide as the reoxidant is advantageous.

The mechanism of OsO4 addition to the olefin was long disputed between [3+2] (proposed by Corey) and [2+2]-then-rearrangement (proposed by Sharpless) mechanisms, but it eventually came to the settlement that the former is more likely.

Corey proposed the following transition state model and has done various tunings of the ligand structure.

-
Examples
The synthesis of zaragozic acid[1]: The Sharpless AD is especially effective for stereoselective synthesis of polyhydroxylated compounds.

-
Experimental Procedure
-
Experimental Tips
Careful handling of osmium is required as it is both toxic and volatile.
-
References
[1] Nicolaou, K. C. et al. Chem. Eur. J. 1995, 1, 467. doi:10.1002/chem.19950010712
-
Related Books