- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
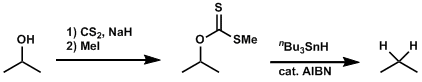
The free radical-mediated deoxygenation of alcohol compounds after the formation of thiocarbonyl intermediates is known as the Barton-MacCombie deoxygenation. This reaction is one of the most popular ways to remove hydroxyl groups.
-
General References
・ Barton, D. H. R.; McCombie, S. W. JCS, Perkin Trans. 1, 1975, 1574. doi:10.1039/P19750001574
・ Crich, D.; Quintero, L. Chem. Rev. 1989, 89, 1413. doi:10.1021/cr00097a001
-
Reaction Mechanism
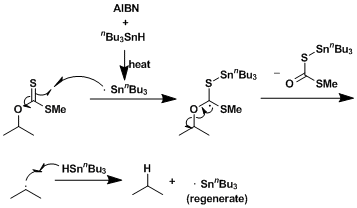
-
Examples
Alkyl xanthates are used commonly, but phenyl thiocarbonates and thiocarbonyl imidazolates are also reactive substrates.[1] 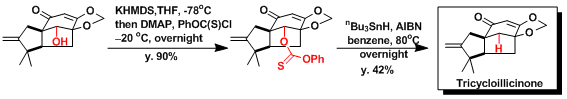
Trapping the radical intermediate is possible when the reacting molecule contains necessary functional groups. An example of intramolecular radical trapping leading to the formation of a five-membered ring is shown here.[2]

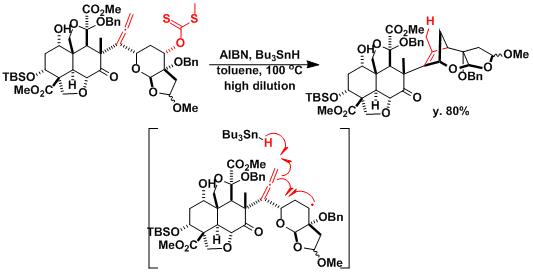
An application found in the total synthesis of azadirachtin.[3]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Danishefsky, S. J. et al. J. Am. Chem. Soc. 2000, 122, 6160. DOI: 10.1021/ja000521m
[2] Tadano, K. et al. J. Org. Chem. 1995, 60, 8179. DOI: 10.1021/jo00130a017
[3] Veitch, G. E.; Beckmann, E.; Burke, B. J.; Boyer, A.; Maslen, S. L.; Ley, S. V. Angew. Chem. Int. Ed. 2007, 46, 7629. DOI:10.1002/anie.200703027
-
Related Books