- Generality
- Reagent Availability
- Experimental User Friendliness
- Historical Significance
- Criteria #5
-
General Characteristics
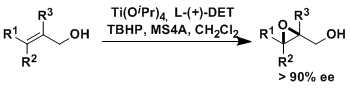
-The asymmetric epoxidation of allylic alcohols using catalytic Ti(OiPr)4, diethyl tartrate (DET), and t-butyl hydroperoxide (TBHP) is known as the Sharpless-Katsuki reaction.
-The absolute configuration of the epoxide products depends only on the absolute configuration of the DET ligand (see the empirical rule shown below). There is no exception to the rule known for prochiral substrates.
-The reaction is generally uninfluenced by the steric factors of the substrates and tolerates a wide range of functional groups well. It can be used for compounds of high complexity and on large scales.
-By using MS3A or 4A, Ti(OiPr)4 and DET can be reduced to catalytic amounts. Water is thought to destroy the active catalytic species.
-Kinetic resolution of racemic secondary allylic alcohols is possible and diisopropyl tartrate is the ligand of choice.
–Z-allylic alcohols tend to be less reactive and less stereoselective.
-The epoxide products are sometimes susceptible to attack by the alkoxides in the system. Using Ti(OtBu)4 in place of Ti(OiPr)4 can improve the yield in this case.
-Barry Sharpless of the Scripps Research Institute was awarded the Nobel Prize in Chemistry in 2001 for the development of this reaction and asymmetric dihydroxylation reaction.
-
General References
・ Katsuki, T.; Sharpless, K. B. J. Am. Chem. Soc. 1980, 102, 5974. DOI: 10.1021/ja00538a077
・ Hanson,, R. M.; Sharpless, K. B. J. Org. Chem. 1986, 51, 1922. DOI: 10.1021/jo00360a058
・ Gao, Y.; Klunder, J. M.; Hanson, R. M.; Masamune, H.; Ko, S. Y.; Sharpless, K. B. J. Am. Chem. Soc. 1987, 109, 5765. DOI: 10.1021/ja00253a032
・ Review: Johnson, R. A.; Sharpless, K. B. Comprehensive Organic Synthesis 1991, 7, 389.
・ Review: Johnson, R. A.; Sharpless, K. B. “Catalytic Asymmetric Synthesis“ Ojima, I. Ed. VCH Publishers, Weinheim, New York, 1993.
・ Review: Katsuki, T.; Martin, V. S. Org. React. 1996, 48, 1.
-
Reaction Mechanism
The active catalytic species is considered to be the titanium dinuclear complex shown below, based on x-ray data of the analogous complex and stereochemical analysis of the products. (ref: J. Am. Chem. Soc. 1984, 106, 6430. J. Am. Chem. Soc. 1991, 113, 106.)
-
Examples
The Sharpless AE reaction is a reliable way to introduce an oxygen atom into molecules enantioselectively. Olefins other than allylic and homoallylic alcohols are generally unreactive under the standard conditions.[1,2]
Achieving high enantioselectivity in asymmetric epoxidation of homoallylic alcohols had been difficult until Yamamoto developed the vanadium-bishydroxamic acid catalyst.[3]
-
Experimental Procedure
An example of reaction procedure.[4]
-
Experimental Tips
TBHP presents a potential explosive hazard. When using it on a large scale, be sure to use a blast shield and conduct the experiment under a fume hood.
-
References
[1][2] Hatakeyama, S. et al. J. Am. Chem. Soc. 1988, 110, 5201. DOI: 10.1021/ja00223a055
[3] Zhang, W.; Yamamoto, H. J. Am. Chem. Soc. 2007, 129, 286. DOI: 10.1021/ja067495y
[4] Gao, Y.; Klunder, J. M.; Hanson, R. M.; Masamune, H.; Ko, S. Y.; Sharpless, K. B.J. Am. Chem. Soc. 1987, 109, 5765.DOI: 10.1021/ja00253a032
-
Related Books
[amazonjs asin=”019850201X” locale=”US” title=”Asymmetric Oxidation Reactions (Practical Approach in Chemistry)”]

![katsuki_sharpless_epoxidation_6[1]](https://en.chem-station.com/wp-content/plugins/lazy-load/images/1x1.trans.gif)