- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
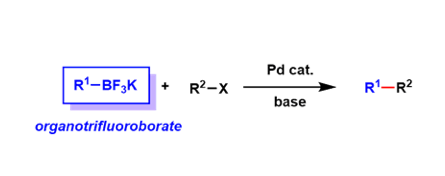
Organotrifluoroborate salts (R-BF3–) are stable towards heat, air, and moisture, and are convenient crystalline reagents.
Because of the tetra-coordinated borate structure, these reagents are not Lewis acidic and are stable under oxidative conditions. They can be regarded as protected forms of boronic acids and boronic acid esters.
These reagents undergo solvolysis in protic solvents to expose tri-coordinated boron species, which can be used for reactions such as the Suzuki-Miyaura coupling. Because they always exist as monomers unlike boronic acids, precise control of stoichiometry is easier.
-
General References
- Chambers, R. D.; Clark, H. C.; Willis, C. J. J. Am. Chem. Soc. 1960, 82, 5298. DOI: 10.1021/ja01505a007
- Vedejs, E.; Chapman, R. W.; Fields, S. C.; Lin, S.; Schrimpf, M. R. J. Org. Chem. 1995, 60, 3020. DOI: 10.1021/jo00115a016
- Vedejs, E.; Fields, S. C.; Hayashi, R.; Hitchcock, S. R.; Powell, D. R.; Schrimpf, M. R. J. Am. Chem. Soc. 1999, 121, 2460. DOI: 10.1021/ja983555r
- Darses, S.; Michaud, G.; Genet, J.-P. Eur. J. Org. Chem. 1999, 1875. [abstract]
- Churches, Q. I.; Hooper, J. F.; Hutton, C. A. J. Org. Chem. 2015, 80, 5428. DOI: 10.1021/acs.joc.5b00182
<reviews>
- Darses, S.; Genet, J.-P. Eur. J. Org. Chem. 2003, 4313. DOI: 10.1002/ejoc.200300294
- Molander, G. A.; Figueroa, R. Aldrichimica Acta 2005, 38, 49. [website]
- Molander, G. A.; Ellis, N. Acc. Chem. Res. 2007, 40, 275. DOI: 10.1021/ar050199q
- Darses, S.; Genet, J.-P. Chem. Rev. 2008, 108, 288. DOI: 10.1021/cr0509758
- Oliveira, R. A. Synlett 2009, 505. DOI: 10.1055/s-0028-1083584
- Molander, G. A.; Canturk, B. Angew. Chem. Int. Ed. 2009, 48, 9240. DOI: 10.1002/anie.200904306
- Lennox, A. J. J.; Lloyd-Jones, G. C. Chem. Soc. Rev. 2014, 43, 412. DOI: 10.1039/c3cs60197h
-
Reaction Mechanism
Despite the tetracoordinated “ate” structure, the strong electronegativity of fluorines make trifluoroborates weak nucleophiles and less reactive in transmetalation.
When used in the Suzuki-Miyaura coupling, it is considered that trifluoroborates are first hydrolyzed to boronic acids and the latter are the actual reactive species. Since the reactive species are slowly released into the system, side reactions such as homo-dimerization tend to be suppressed. (Ref: Angew. Chem. Int. Ed. 2010, 49, 5156.)
-
Examples
Tetraalkylammonium borate salts are more soluble in nonpolar organic solvents.[1]

An application to the coupling of unprotected peptides.[2]

The selection of a right solvent enabled the chemoselective Heck reaction of vinyl trifluoroborate, which was followed by the Suzuki coupling.[3]

The concise synthesis of (+)-frondosin B[4]: The asymmetric conjugate addition using the MacMillan catalyst[5] was the key step.

An example of trifluoromethylation using a visible light redox catalyst system.[6]

-
Experimental Procedure
Preparation of organotrifluoroborate salts.[7]

Phenylboronic acid (20 g, ca. 169 mmol) was dissolved in 50 mL of methanol. Excess saturated KHF2 (125 mL, ca. 4.5 M solution, ca. 563 mmol) was added slowly with vigorous stirring. After 15 min, the precipitated product was collected and washed with cold methanol. Recrystallization from minimal acetonitrile produced 25.5 g (138 mmol, 82%) of pure potassium phenyltrifluoroborate.
-
Experimental Tips
-
References
- Batey, R. A.; Quach, T. D. Tetrahedron Lett. 2001, 42, 9099. doi:10.1016/S0040-4039(01)01983-9
- (a) Noda, H.; Eros, G.; Bode, J. W. J. Am. Chem. Soc. 2014, 136, 5611. DOI: 10.1021/ja5018442
- Molander, G.A.; Sandrock, D. L. Org. Lett. 2009, 11, 2369. DOI: 10.1021/ol900822j
- Reiter, M.; Torssell, S.; Lee, S.; MacMillan D. W. C. Chem. Sci. 2010, 1, 37. DOI: 10.1039/c0sc00204f
- Lee, S.; MacMillan, D. W. C. J. Am. Chem. Soc. 2007, 129, 15438. DOI: 10.1021/ja0767480
- Yasu, Y.; Koike, T.; Akita, M. Chem. Commun. 2013, 49, 2037. DOI: 10.1039/C3CC39235J
- Vedejs, E.; Chapman, R. W.; Fields, S. C.; Lin, S.; Schrimpf, M. R. J. Org. Chem. 1995, 60, 3020. DOI: 10.1021/jo00115a016
-
Related Reactions
- 鈴木-宮浦クロスカップリング (Suzuki-Miyaura Coupling)
- ボロン酸MIDAエステル (Boronic Acid MIDA Ester)
-
Related Books
[amazonjs asin=”B00EHKTLPY” locale=”US” title=”Organotrifluoroborate Preparation, Coupling and Hydrolysis (Springer Theses)”]
[amazonjs asin=”3527325980″ locale=”US” title=”Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials”]
-
External Links
- Organotrifluoroborate – Wikipedia
- Organotrifluoroborate (Sigma-Aldrich)