- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
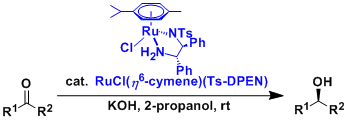
The asymmetric “transfer hydrogenation” of ketones is catalyzed by Ru(II)-arene-tosyldiamine and related complexes. Some of these ruthenium complexes are commercially available and they are stable enough to be handled easily in open air.
Under 2-propanol-KOH conditions, 2-propanol works as the hydrogen source. The combination of HCO2H-Et3N is also used frequently.
-
General References
・Hashiguchi, S.; Fujii, A.; Takehara, J.; Ikariya, T.; Noyori, R. J. Am. Chem. Soc. 1995, 117, 7562. DOI: 10.1021/ja00133a037
・Fujii, A.; Hashiguchi, S.; Uematsu, N.; Ikariya, T.; Noyori, R. J. Am. Chem. Soc. 1996, 118, 2521. DOI: 10.1021/ja954126l
・Haack, K.-J.; Hashiguchi, S.; Fujii, A.; Ikariya, T.; Noyori, R. Angew. Chem. Int. Ed. 1997, 36, 285. doi:10.1002/anie.199702851
<Mechanism>
・ Yamakawa, M.; Ito,H.; Noyori, R. J. Am. Chem. Soc. 2000, 122, 1466. DOI: 10.1021/ja991638h
・ Yamakawa, M.; Yamada, I.; Noyori, R. Angew. Chem. Int. Ed. 2001, 40, 2818. [abstract]
・ Noyori, R.; Yamakawa, M.; Hashiguchi, S. J. Org. Chem. 2001, 66, 7931. DOI: 10.1021/jo010721w
-
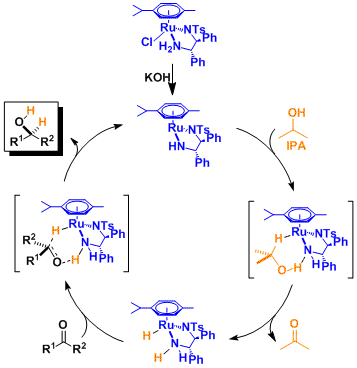
Reaction Mechanism
Similar to the hydrogenation catalyzed by Ru(II)-diphosphine-diamine complexes (see the Noyori asymmetric hydrogenation), the hydrogen atom of the amine is believed to interact with the substrate, playing a key role in enhancing both reactivity and stereoselectivity (the Noyori-Ikariya model).
-
Examples
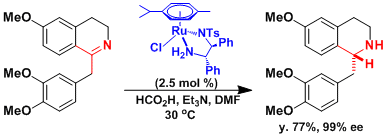
The asymmetric C=N reduction in the synthesis of morphine.[1]
-
Experimental Procedure
Synthesis of optically active hydrobenzoin from racemic benzoin via dynamic kinetic resolution.[2]
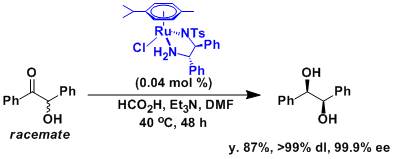
A 1 L four-necked, round-bottomed flask equipped with a mechanical stirrer, a reflux condenser bearing an inert gas inlet tube, a thermometer and a dropping funnel is charged with 290 mL (2.08 mol) of triethylamine. The triethylamine is cooled to 4 °C in an ice bath and formic acid (97.0 mL, 2.57 mol) is added slowly. To the mixture of formic acid and triethylamine at ambient temperature is added rac-benzoin (170 g, 0.801 mol), RuCl[1S,2S-N-p-toluenesulfonyl-1,2-diphenylethanediamine]-(η6–p-cymene) (0.204 g, 0.321 mmol), and dry DMF (80 mL) . After the reaction mixture is stirred at 40 °C for 48 h, 300 mL of water is added at 0 °C with stirring. The pale pink precipitate is filtered through a Büchner funnel, washed with water (2 x 500 mL), and dried in vacuo to give a white solid in 97% yield. The crude product is dissolved in hot methanol (700 mL) at 60 °C. A small amount of insoluble material is removed through filtration and the filtrate is cooled initially to room temperature and then to 0 to 5 °C to provide white crystals. The crystalline product is isolated by filtration, washed with cooled (ice bath) 2-propanol (400 mL), and dried to provide 129.7 g of optically pure (R,R)-hydrobenzoin as white crystals (dl > 99%, 99.9% ee). Concentration of the mother liquors and another recrystallization from methanol (100 mL) provides a second crop of the product, 19.1 g (dl > 99%, 99.9% ee). The overall yield is 148.8 g (87%).
-
Experimental Tips
-
References
[1] Meuzelarr, G. J.; Van Vliet, M. C. A.; Maat, L.; Sheldon, R. A. Eur. J. Org. Chem. 1999, 2315. [abstract]
[2] Ikariya, T.; Hashiguchi, S.; Murata, K.; Noyori, R. Org. Synth. 2005, 82, 10. [PDF]
-
Related Books