- Generality
- Reagent Availability
- Experimental User Friendliness
- Industrial Importance
- Historical Significance
-
General Characteristics
The asymmetric hydrogenation of olefins and ketones catalyzed by Ru(II)-BINAP and related ruthenium complexes is called the Noyori hydrogenation.
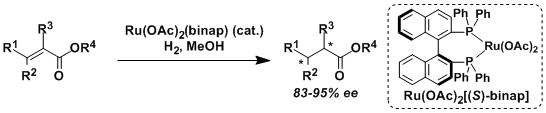
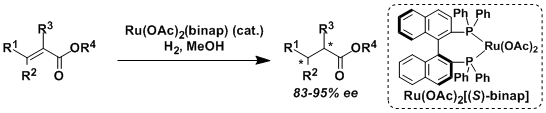
The hydrogenation of olefins and ketones using Ru(OAc)2(binap) and RuCl2(binap)(dmf)n requires the coordination of neighboring functional groups such as ester, amide, carboxylic acid, and alcohol.
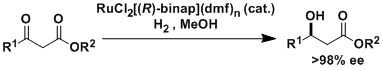
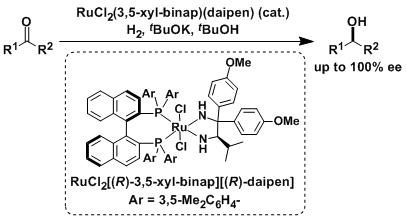
The CBS reduction used to be the only general solution to achieve catalytic asymmetric reduction of simple unfunctionalized ketones. In the 1990’s, Noyori’s group discovered that Ru(II)-diphosphine-diamine complexes were capable of catalyzing asymmetric hydrogenation of simple ketones. This hydrogenation is highly chemoselective that ketones (and aldehydes) can be reduced in the presence of olefins. The catalytic activity of these catalysts is also very high. Depending on the substrate, the catalytic turnover (TON) exceeds 100,000.
For its substrate generality, cleanness, and high atom economy, the Noyori hydrogenations find many applications in industrial processes. Professor Ryoji Noyori was the recipient of the Nobel Prize in Chemistry in 2001 for his contributions to the development of catalytic asymmetric reduction.
-
General References
・Noyori, R. Asymmetric Catalysis in Organic Synthesis , Ojima, I. Ed.; John Wiley & Sons; New York, 1994, chapter 2.
・Noyori, R..; Ohkuma, T. Angew. Chem., Int. Ed. 2001, 40, 40. [abstract]
・大熊 毅, 有機合成化学協会誌 2001, 59, 446.
・塚本真幸, 北村雅人, 有機合成化学協会誌 2005, 63, 899.
<Nobel Lectures>
・Knowles, W. S. Angew. Chem. Int. Ed. 2001, 41, 1998. [abstract]
・Noyori, R. Angew. Chem. Int. Ed. 2001, 41, 2008. [abstract]
-
Reaction Mechanism
The oxidation state of the ruthenium remains+2 within the catalytic cycle, unlike that of rhodium catalysts that changes back and forth between +1 and +3.
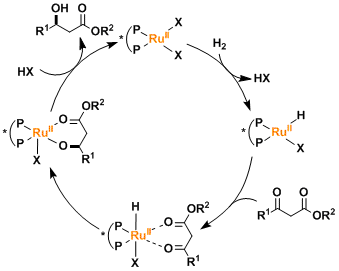
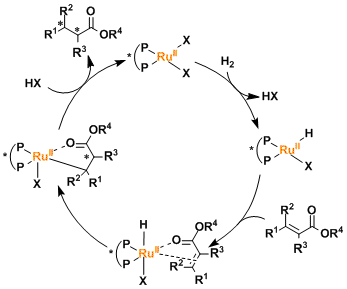
In Ru(II)-diphosphine-diamine catalytic systems, the presence of a hydrogen atom on the amine is essential and believed to play a role of stabilizing the catalyst-substrate interaction in the transition state. (See the Noyori asymmetric transfer hydrogenation.)
-
Examples

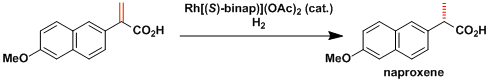
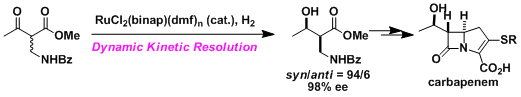
-
Experimental Procedure
Asymmetric synthesis of (R)-citronellol from geraniol.[1]
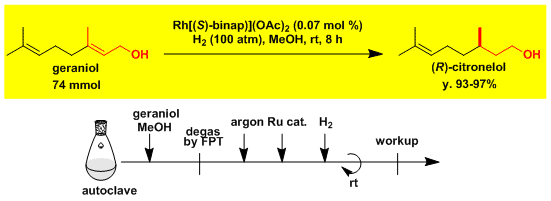
-
Experimental Tips
-
References
[1] Org. Synth. 1993, 72, 74.
-
Related Books
[amazonjs asin=”3527311610″ locale=”US” title=”Handbook of Homogeneous Hydrogenation (3 Volumes)”]
[amazonjs asin=”047017577X” locale=”US” title=”Catalytic Asymmetric Synthesis”]
[amazonjs asin=”3527306927″ locale=”US” title=”Ruthenium in Organic Synthesis”]
[amazonjs asin=”3527324895″ locale=”US” title=”Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions”]