- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
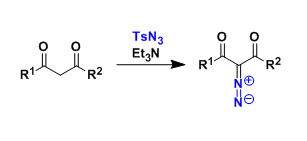
Activated methylene compounds react with sulfonyl azides and related reagents to give the corresponding diazo compounds.
These diazo compounds are used as 1,3-dipoles or precursors of metal carbenoids. In the latter case, the metal carbenoids are used for such reactions as cyclopropanation and C-H insertion.
-
General References
Dimroth, O. et al. Ann. 1910, 373, 336.
Regitz, M. Ann. 1964, 676, 101.
Regitz, M. Tetrahedron Lett. 1964, 5, 1403. doi:10.1016/S0040-4039(00)90489-1
Regitz, M.; Heck, G. Ber. 1964, 97, 1482.
Regitz, M. Ber. 1964, 97, 2742.
Regitz, M.; Anschuetz, W.; Bartz. W.; Liedhegener, A. Tetrahedron Lett. 1968, 9, 3171. doi:10.1016/S0040-4039(00)89580-5
Regitz, M. Angew. Chem. Int. Ed. Engl. 1967, 6, 733. doi:10.1002/anie.196707331
Regitz, M. Synthesis 1972, 351. DOI: 10.1055/s-1972-21883
Ye, T.; McKervey, M. A. Chem. Rev. 1994, 94, 1091. DOI: 10.1021/cr00028a010
Maas, G. Angew. Chem. Int. Ed. 2009, 48, 8186. doi:10.1002/anie.200902785
-
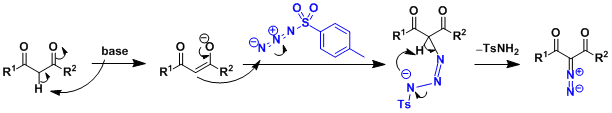
Reaction Mechanism
-
Examples
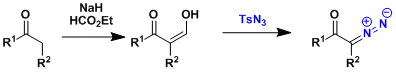
The diazo transfer does not occur at the α-position of simple ketones, and in those cases, the pre-introduction of formyl group is an effective approach.[1] Trifluroacetyl group can be used for the same purpose for more sensitive compounds.[2]
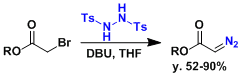
Inexpensive and readily synthesized N,N’-bistosylhydrazine is a useful reagent for the conversion of α-haloesters to α-diazoesters.[3]
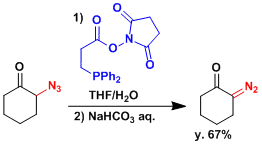
The reagent that can convert azide to diazo group in a single step has been developed.[4]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Regtiz, M.; Rueter, J. Chem. Ber. 1968, 101, 1263. [2] Danheiser, R. L.; Miller, R. F.; Brisbois, R. G.; Park, S. Z. J. Org .Chem. 1990, 55, 1959. DOI: 10.1021/jo00293a053 [3] Toma, T.; Shimokawa, J.; Fukuyama, T. Org. Lett. 2007, 9, 3195. DOI: 10.1021/ol701432k [4] Myers, E. L.; Raines, R. T. Angew. Chem. Int. Ed. 2009, 48, 2359. DOI:10.1002/anie.200804689
-
Related Reactions
Cyclopropanation with Metal Carbenoid
C-H Insertion of Metal Carbenoid
Seyferth-Gilbert Alkyne Synthesis
-
Related Books
-
External Links