Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
Sodium bis(2-methoxyethoxy)aluminum hydride is a reducing agent sold with the common name Red-Al or Vitride.
Red-Al can selectively reduce nitriles to aldehydes. It is more soluble in organic solvents (such as toluene) than LAH. It is also more thermally stable and not pyrophoric.
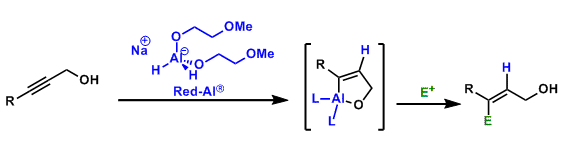
As shown in the scheme above, it can be used to reduce propargyl alcohols to allyl alcohols via hydrometallation. The alkenylaluminum intermediate is reactive towards various electrophiles, thus can be functionalized further. Unlike DIBAL, Red-Al gives trans-substituted products.
-
General References
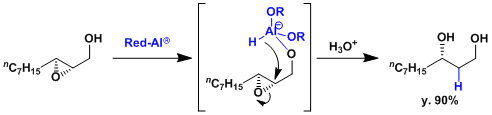
Substrate-directed regioselective ring opening of epoxide.
-
Reaction Mechanism
[1] Katsuki, T.; Martin, V. S. Org. React. 1996, 48, 1.
-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Reactions
- L/N/K-Selectride
- Schwartz’s Reagent
- Sodium Borohydride
- Diisobutylaluminium hydride
- Lithium Alminum Hydride (LAH)
- Hydrometalation
- Partial Reduction of Esters, Amides nad Nitriles with Metal Hydride
- Brown Hydroboration
-
Related Books
-
External Links
- Red-Al (organic-chemistry.org)