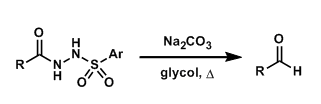- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
The thermal decomposition of acylarylsulfonylhydrazines into the corresponding aldehydes under basic conditions is known as the McFadyen-Stevens reaction. The reaction has been modified to effect the transformation under mild conditions.
-
General References
・McFadyen, J. S.; Stevens, T. S. J. Chem. Soc. 1936, 584.
・Mosettig, E. Org. React. 1954, 8, 232.
・Dudman, C. C.; Grice, P.; Reese, C. B. Tetrahedron Lett. 1980, 21, 4645. doi:10.1016/0040-4039(80)80096-7
・Iwai, Y.; Ozaki, T.; Takita, R.; Uchiyama, M.; Shimokawa, J.; Fukuyama, T. Chem. Sci. 2012, doi:10.1039/C2SC22045H
-
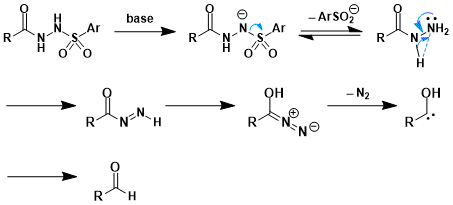
Reaction Mechanism
(Reference: Chem.Sci. 2012.)
-
Examples
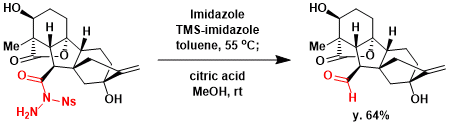
Under the conditions modified by Fukuyama, the reaction of N,N-acylsulfonyl hydrazine proceeds at lower temperature than the original conditions.[1]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Iwai, Y.; Ozaki, T.; Takita, R.; Uchiyama, M.; Shimokawa, J.; Fukuyama, T. Chem. Sci. 2012, doi:10.1039/C2SC22045H
-
Related Reactions
- Wharton Reaction
- Movassaghi Deoxigenation
- Shapiro Reaction
- Eschenmoser-Tanabe Fragmentation
- Wolff-Kishner Reduction
-
Related Books
-
External Links