- Generality
- Principles of Organic Synthesis
- Criteria #3
- Criteria #4
- Criteria #5
-
General Characteristics
The rate of ring closing reactions is largely influenced by how easily the orbitals can interact and overlap at the reacting parts of the molecule. The empirical rule that summarizes the relative rates of various cyclization types is known as Baldwin’s rules. This set of rules is applicable to most reactions including nucleophilic, electrophilic and free-radical reactions.
-
General References
- Baldwin, J. E. J. Chem. Soc. Chem. Commun. 1976, 18, 734. doi:10.1039/C39760000734
- Baldwin, J. E., et al. J. Org. Chem.1977, 42, 3846. doi:10.1021/jo00444a011
- Baldwin, J. E.; Lusch, M. J. Tetrahedron 1982, 38, 2939. doi:10.1016/0040-4020(82)85023-0
- Chatgilialoglu, C.; Ferreri, C.; Guerra, M.; Timokhin, V.; Froudakis, G.; Gimisis, T. J. Am. Chem. Soc. 2002, 124, 10765. DOI: 10.1021/ja0261731
-
History
In 1976, the rules regarding the relative ease of cyclization were summarized and reported by Jack Baldwin at Oxford University.
-
Details
Taking entropic, stereoelectronic, and ring strain effects into consideration, general empirical rules can be summarized as follows:
・5~7 membered rings are easiest to form.
・3 and 4 membered rings are slower to form due to ring strain.
・8~11 membered rings are most difficult to form.
・Formation of 12 and higher membered rings is entropically disfavored and thus intermolecular reaction tends to compete.
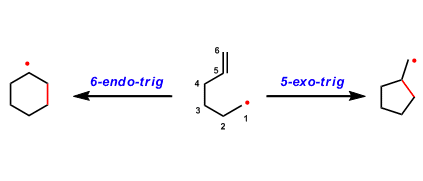
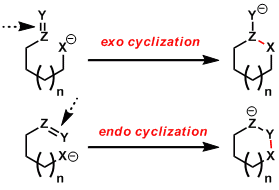
Cyclization types are distinguished by exo and endo cyclizations. When the breaking bond is positioned external to the ring being formed, it is called exo cyclization. On the other hand, when the breaking bond is inside of the ring being formed, it is called endo cyclization.
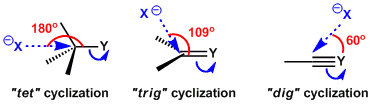
The hybridization of the atom being attacked is also classified into three types, tet, trig, and dig, depending on whether the atom is sp3, sp2 or sp hybridized, respectively. Since the formation of a new bond takes place when orbitals can overlap, the rate of cyclization is determined by how efficiently the reacting atoms approach with each other with an appropriate angle.
Adding the number of atoms in the ring to the above classifications, the rules are summarized as follows:
・Favored: 3~7-exo-tet, 3~7-exo-trig, 3,4-exo-dig
・Disfavored: 5,6-endo-tet, 3~5-exo-trig, 3~7-endo-dig
Note that “disfavored” does not mean those cyclizations do not occur, but they are simply slower to occur than favored cyclizations.
-
Reaction Mechanism
-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Reactions
Intramolecular Radical Cyclization
-
Related Books
-
External Links