Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
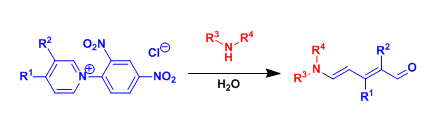
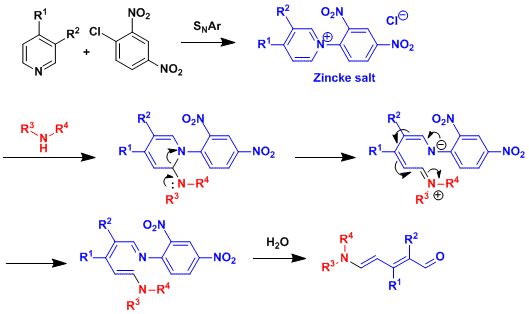
Upon nucleophilic attack by secondary amines, 1-(2,4-dinitrophenyl)pyridinium chloride (the Zincke salt) opens up to give 5-aminopenta-2,4-dienal (the Zincke aldehyde).
-
General References
- Zincke, T. H.; Heuser, G.; Moller, W. Liebigs Ann. Chem. 1904, 333, 296. doi:10.1002/jlac.19043330212
- Zincke, T. H.; Heuser, G.; Moller, W. Liebigs Ann. Chem. 1904, 330, 361. doi:10.1002/jlac.19043300217
- Zincke, T. H.; Weisspfenning, G. Liebigs Ann. Chem. 1913, 396, 103. doi:10.1002/jlac.19133960107
- Becher, J. Synthesis 1980, 589. DOI: 10.1055/s-1980-29134
- Becher, J.; Finsen, L.; Winckelmann, I. Tetrahedron 1981, 37, 2375. doi:10.1016/S0040-4020(01)88892-X
- Cheng, W.-C.; Kurth, M. J. Org. Prep. Proced. Int. 2002, 34, 587.
-
Reaction Mechanism
-
Examples
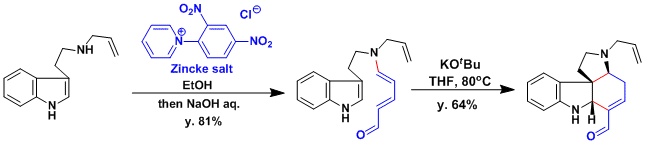
The aminodienal prepared using the Zincke salt served as a key intermediate in the synthesis of a complex indole alkaloid.[1]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] (a) Martin, D.B.C.; Vanderwal, C.D. J. Am. Chem. Soc. 2009, 131 , 3472. doi:10.1021/ja900640v
(b) Martin, D.B.C.; Vanderwal, C.D. Chem. Sci. 2011, 2, 649. doi:10.1039/C1SC00009H
-
Related Reactions
-
Related Books
-
External Links