- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
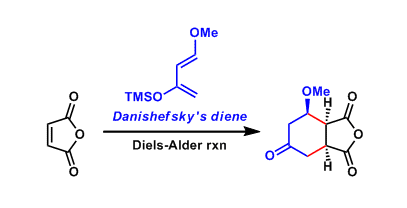
trans-1-Methoxy-3-trimethylsiloxy-1,3-butadiene is commonly called the Danishefsky-Kitahara diene (or simply Danishefsky diene). The high HOMO energy level resulting from two electron-donating groups makes it a highly reactive reagent in the Diels-Alder and hetero Diels-Alder cycloadditions.
-
General References
- Danishefsky, S. J.; Kitahara, T. J. Am. Chem. Soc. 1974, 96, 7807. doi:10.1021/ja00832a031
- Danishefsky, S. J.; Hirama, M.; Gombatz, K.; Harayama, T.; Berman, E.; Schuda, P. J. Am. Chem. Soc. 1978, 100, 6536. DOI: 10.1021/ja00488a063
- Danishefsky, S. J. Acc. Chem. Res. 1981,14, 400. DOI: 10.1021/ar00072a006
- Danishefsky, S. J.; Kitahara, T.; Shuda, P. F. Org. Synth. 1983, 61, 147. [website]
-
Reaction Mechanism
-
Examples
-
Experimental Procedure
Preparation of the Danishefsky diene.[1]
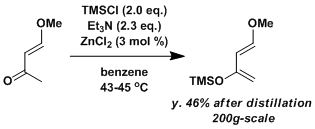
Triethylamine (575 g, 5.7 mol) is stirred mechanically in a three-necked flask. To this is added 10.0 g (0.07 mol) of pre-dried zinc chloride. The mixture is stirred at room temperature under nitrogen for 1 hr. A solution of 250 g (2.50 mol) of 4-methoxy-3-buten-2-one in 750 mL of benzene is added all at once. Mechanical stirring is continued for 5 min. Chlorotrimethylsilane (542 g, 5.0 mol) is added rapidly. The reaction mixture first turns pink, then red, and finally brown. Heat is evolved and the reaction is kept below 45°C by cooling in an ice bath. After 30 min, the mechanically stirred solution is heated by a heating mantle to 43°C. This temperature is maintained for 12 hr. The reaction mixture becomes very thick during this time. After the mixture cools to ambient temperature, it is poured, with mixing, into 5 L of ether. The solid material is filtered through Celite. The Celite and solid material are removed and stirred with 4 L more of ether and refiltered through Celite. The combined ether washings are evaporated under reduced pressure to a brown, sweet-smelling oil. The oil is transferred to a 1-L, single-necked flask equipped with an 18-in. Vigreux column. Careful fractional distillation under water vacuum affords a forerun of approximately 16 g that boils at 70–78°C (22 mmHg). This fraction consists of impure diene that contains 4-methoxy-3-buten-2-one. The main fraction boils at 78–81°C (23 mmHg) and consists of 245 g of diene with approximately 5–10% of 4-methoxy-3-buten-2-one. This material is suitable for most purposes. If higher purity is desired, the second fraction may be redistilled under reduced pressure through an 18-in. Vigreux column to afford 200 g (46%) of trans-1-methoxy-3-trimethylsiloxy-1,3-butadiene.
-
Experimental Tips
It is important to use pre-dried anhydrous zinc chloride.
-
References
[1] Danishefsky, S. J.; Kitahara, T.; Shuda, P. F. Org. Synth. 1983, 61, 147. [website]
-
Related Reactions
- Asymmetric Diels-Alder Reaction
- Hetero Diels-Alder Reaction
- Mukaiyama Aldol Reaction
- Diels-Alder Reaction
-
Related Books
-
External Links