- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
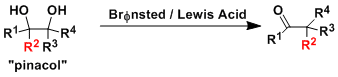
Vicinal diol compounds (pinacols) undergo dehydrative rarrangement under strongly acidic conditions to produce carbonyl compounds.
Since alkyl, aryl, and hydride are all potential migrating groups, pinacols containing different R1~R4 groups are likely to give a mixture of products.
In general, more electron rich groups migrate more easily. A rough order for the propensity to migrate is: aryl > H > alkenyl > tert-alkyl >> cyclopropyl > sec-alkyl > prim-alkyl.
Vicinal diols with one of the hydroxyl groups activated as a leaving group undergo the same rearrangement and this version is called the semi-pinacol rearrangement. This reaction proceeds under milder conditions and is synthetically valuable if the starting materials are appropriately designed.
-
General References
・ Fittig, R. Liebigs Ann. Chem. 1860, 114, 54.
・ Rickborn, B. Comp. Org. Syn. 1991, 3, 721.
-
Reaction Mechanism
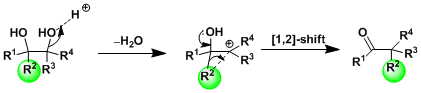
The reaction proceeds by the cationic 1,2-shift mechanism similar to the Wagner-Meerwein rearrangement. The rearrangement is promoted by the presence of a lone pair of the neighboring hydroxyl group. When the substrate forms a stable carbocationic intermediate and bond rotation competes with rearrangement, the stereochemistry of the product becomes less predictable.
-
Examples
Pinacols containing cyclic groups can be considered as precursors of spiro compounds. The pinacol rearrangement of these cyclic substrates is a typical example of ring-expansion reactions.

-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Books