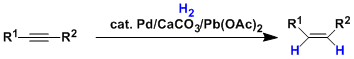- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
The palladium catalyst supported on calcium carbonate and partially deactivated (“poisoned”) with lead acetate and quinoline is called the Lindlar catalyst.
Using the Lindlar catalyst, the hydrogenation of alkynes stops at alkenes without further hydrogenation to alkanes. This chemoselectivity is explained by the stronger adsorption of alkynes to the catalyst surface than alkenes. The hydrogenation is also stereoselective and Z-alkenes are produced as a major product.
-
General References
・Lindlar, H. Helv. Chim. Acta 1952, 35, 446. doi:10.1002/hlca.19520350205
-
Reaction Mechanism
-
Examples
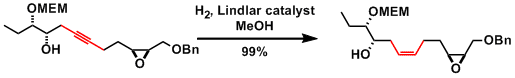
Example 1.[1]
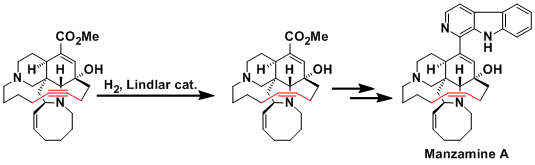
The synthesis of manzamine A.[2]
-
Experimental Procedure
-
Experimental Tips
The chemoselectivity is sensitive to various factors and not always perfect. One needs to monitor the reaction carefully.
-
References
-
Related Books
[amazonjs asin=”B00IGYNTAU” locale=”US” title=”Oxidation and Reduction in Organic Synthesis (Oxford Chemistry Primers) by Donohoe, Timothy J. (2000) Paperback”]
[amazonjs asin=”0471396982″ locale=”US” title=”Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis”]