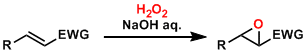- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
Electron-deficient olefins are epoxidized by oxidants such as hydrogen peroxide and t-butylhydroperoxide (TBHP) under basic conditions. Olefins without electron-withdrawing functional groups are unreactive because the reaction proceeds by nucleophilic addition of the oxidants.
-
General References
・Julia, S.; Masana, J.; Vega, J. Angew. Chem. Int. Ed., Engl. 1980, 19, 929. doi:10.1002/anie.198009291
・Banfi, S.; Colonna, S.; Molinari, H.; Julia Guixer, J. Tetrahedron 1984, 40, 5207. doi:10.1016/S0040-4020(01)91272-4
・House, H. O.; Ro, R. S. J. Am. Chem. Soc. 1958, 80, 2428. DOI: 10.1021/ja01543a021
-
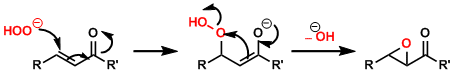
Reaction Mechanism
The nucleophilic 1,4-addition of peroxide is followed by three-membered ring formation with the elimination of hydroxide or alkoxide. Note the mechanistic difference between this and the oxidation of electron-rich olefins with mCPBA.
-
Examples
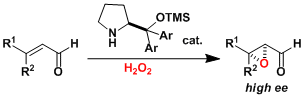
Asymmetric epoxidation of α,β-unsaturated aldehydes with an organocatalyst.[1]
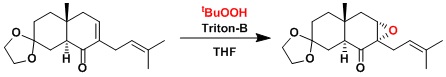
An example of chemoselective epoxidation[2]: The prenyl group is not oxidized under these conditions.
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Marigo,M.; Franzen, J.; Poulsen, T. B.; Zhuang, W.; Jorgensen, K. A. J. Am. Chem. Soc. 2005, 127, 6964. DOI: 10.1021/ja051808s
[2] Grieco, P. A.; Nishizawa, M.; Oguri, T.; Burke, S. D.; Marinovic, N. J. Am. Chem. Soc. 1977, 99, 5773. DOI: 10.1021/ja00459a039
-
Related Books