- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
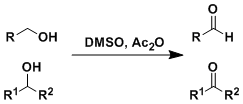
The Swern-type oxidation of alcohols using acetic anhydride as the activator of DMSO. The reaction times are relatively long (12-24 h). This reaction is useful for the oxidation of sterically hindered positions.
-
General References
・Albright, J. D.; Goldman, L. J. Am. Chem. Soc. 1965,87, 4214. DOI: 10.1021/ja01096a055
・Albright, J. D.; Goldman, L. J. Am. Chem. Soc. 1967,89, 2416. DOI: 10.1021/ja00986a031
・Review: Tidwell, T. T. Synthesis 1990, 857. DOI: 10.1055/s-1990-27036
-
Reaction Mechanism
The basic mechanism is same as that of the original Swern oxidation. ![albright_goldman_2[1]](https://assets.en.chem-station.com/uploads/2014/07/albright_goldman_21.gif)
-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Broka, C. A.; Gerlits, J. F. J. Org. Chem. 1988, 53, 2144. DOI: 10.1021/jo00245a002
-
Related Books
[amazonjs asin=”0198556640″ locale=”US” title=”Oxidation and Reduction in Organic Synthesis (Oxford Chemistry Primers, 6)”] [amazonjs asin=”3527306420″ locale=”US” title=”Modern Oxidation Methods”] [amazonjs asin=”0387236074″ locale=”US” title=”Oxidation of Alcohols to Aldehydes and Ketones: A Guide to Current Common Practice (Basic Reactions in Organic Synthesis)”]

![albright_goldman_3[1]](https://assets.en.chem-station.com/uploads/2014/07/albright_goldman_31.gif)