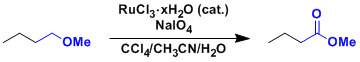- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
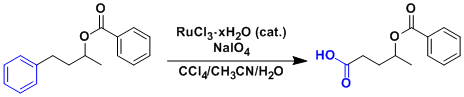
-
General Characteristics
Ruthenium tetroxide is a very strong oxidizing agent capable of even oxidative cleavage of benzene rings and olefins. The reagent can therefore be thought of as an alternative of ozonolysis. However, the strongly oxidizing conditions have low functional group tolerance and can cause unwanted side reactions. One needs to be careful about when to use it and the compatibility of the substrate.
-
Reaction Mechanism
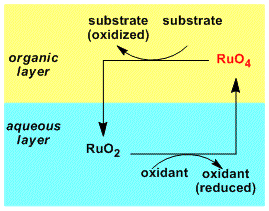
Because ruthenium is relatively expensive, it is common to use the combination of a catalytic amount of ruthenium source and an inexpensive reoxidant that produce Ru(VIII) in situ. For the solvent, carbon tetrachloride is usually used as it is inert to the reagent. Other solvents such as ethers are too reactive to be used. The coordination of the substrate and the product tend to deactivate the catalyst, but it can be prevented by adding acetonitrile as a coordinating cosolvent. For these reasons, the biphasic conditions consisting of RuCl3(cat.)-NaIO4/CH3CN-CCl4-H2O are used most frequently.
Like the scheme shown above, electron-poor aromatic rings are usually unreactive.
-
General References
-
Reaction Mechanism
RuO4 (soluble in organic solvents) is consumed by the reaction and moves into the aqueous layer as RuO2 (soluble in water), where it is oxidized back to RuO4 by the stoichiometric reoxidant.
-
Examples
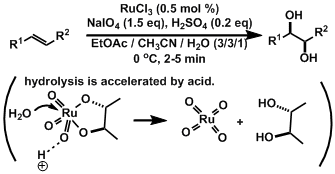
Cis-dihydroxylation of olefins.[1]
These conditions do not require toxic osmium reagent. The acidity of the system is important. The reactions times are very short.
An application to the deprotection of MOM and Bn ethers.
The deprotection of stable ether protecting groups such as MOM and Bn usually require strongly acidic conditions, which is often problematic in late stages of total synthesis.
These ethers can be oxidized to methyl carbonate and benzoyl ester, respectively, which can be deprotected under milder conditions.
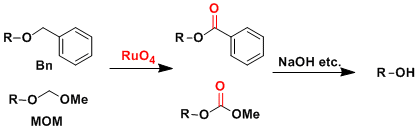
-
Experimental Procedure
Oxidative cleavage of a benzene ring.[2]
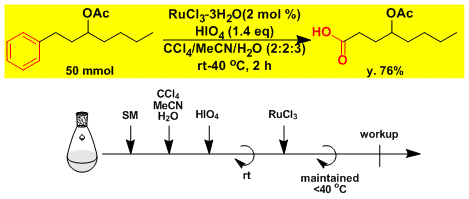
-The reaction produces CO2 gas, so closed systems should not be used.
-The control of reaction temperature (25-40℃) is important.
-The ruthenium residue can be removed by a short pad of silica gel.
-
Experimental Tips
-
References
[1] (a) Plietker,B.; Niggemann, M.; Org. Lett. 2003, 5, 3353. DOI: 10.1021/ol035335a (b) Plietker,B.; Niggemann, M.; Pollrich, A. Org. Biomol. Chem. 2004, 2, 1116. DOI: 10.1039/b316546a
[2] Teresa Nunez, M.; Martin, V. S. J. Org. Chem. 1990, 55, 1928. DOI: 10.1021/jo00293a044
-
Related Books
[amazonjs asin=”3527306420″ locale=”US” title=”Modern Oxidation Methods”]
[amazonjs asin=”3540205438″ locale=”US” title=”Ruthenium Catalysts and Fine Chemistry (Topics in Organometallic Chemistry)”]