- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
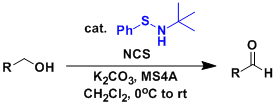
The Swern oxidation is relatively clean and practical but also associated with such problems as the generation of carbon monoxide and dimethylsulfide and the requirement of cryogenic temperature. Hypervalent iodine reagents such as the Dess-Martin reagent are less problematic but nevertheless have their own problem (potential risks of explosion).
The Mukaiyama oxidation catalyzed by the benzenesulfenamide is one of the oxidation methods developed more recently to overcome the problems. The reaction proceeds using easily handleable reagents and under mild conditions (room temperature, weakly basic conditions).
Similar to the oxidations using TPAP and TEMPO, the Mukaiyama oxidation is catalytic and amenable to large scale synthesis.
-
General References
・Mukaiyama, T.; Matsuo, J.-i.; Yanagisawa, M. Chem. Lett. 2000, 1072, doi:10.1246/cl.2000.1072
・Mukaiyama, T.; Matsuo, J.-i.; Kitagawa, H. Chem. Lett. 2000, 1250. doi:10.1246/cl.2000.1250
・Matsuo, J.-i.; Kitagawa, H.; Iida, D.; Mukaiyama, T. Chem. Lett. 2001, 150, doi:10.1246/cl.2001.150
・Mukaiyama, T.; Matsuo, J.-i.; Iida, D.; Kitagawa, H. Chem. Lett. 2001, 846. doi:10.1246/cl.2001.846
・Matsuo, J.-i.; Iida, D.; Tatani, K.; Mukaiyama, T. Bull. Chem. Soc. Jpn. 2002, 75, 223. doi:10.1246/bcsj.75.223
・Mukaiyama, T. et al. Tetrahedron 2003, 59, 6739. doi:10.1016/S0040-4020(03)00479-4
・有機合成化学協会誌 2004, 62, 574.
・Recent Review on Mukaiyama’s Work: Angew. Chem. Int. Ed. 2004, 43, 5590. doi:10.1002/anie.200300641
-
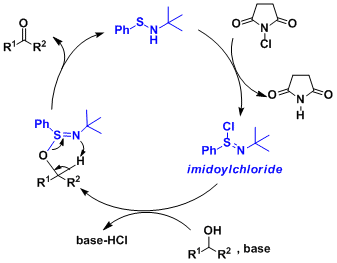
Reaction Mechanism
The active species formed in situ is the imidoyl chloride, which undergoes alcohol addition and intramolecular deprotonation.
-
Examples
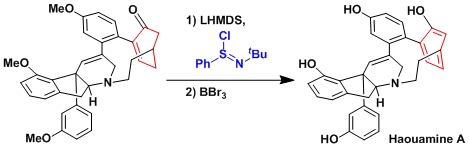
α,β-Unsaturated carbonyl compounds can be synthesized by the oxidation of lithium enolates with stoichiometric imidoyl chloride. Shown below is an example used in the total synthesis of haouamine A.[1]
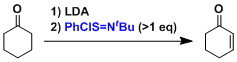
An application to the middle stage in the total synthesis of taxol[2]: The oxidation proceeds without damaging functional groups.
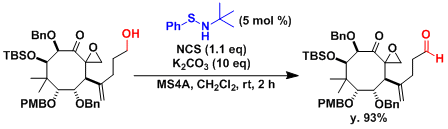
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Burns, N. A.; Krylova, I. N.; Hannoush, R. N.; Baran, P. S. J. Am. Chem. Soc. 2009, 131, 9172. doi:10.1021/ja903745s
[2] Mukaiyama, T. et al. Chem. Eur. J. 1999, 5 , 121. [abstract]
-
Related Books