- atom economical
- Responsibility
- Criteria #3
- Criteria #4
- Criteria #5
-
Characteristics
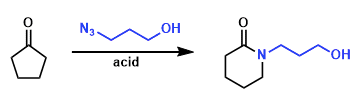
Boyer-Schmidt-Aube Rearrangement is the Lewis acid-mediated reactions of simple ketones with alkyl azides. It has been shown that the Schmidt reaction of alkyl azides, a reaction proposed over 50 years ago, can occur but is only synthetically useful for a fairly restricted set of carbonyl-containing substrates.
In 1951, Boyer and co-workers did manage to establish a narrow range of azides that react with aromatic aldehydes, but the bona fide migration of an alkyl group was not observed in any of these example.[a] In addition, the intramolecular reaction of several enones with azides gave Schmidt-type products upon thermolysis, but the reaction proceeds by initial attack of the azide upon the double bond followed by rearrangement of the resulting triazoline.
An intramolecular Schmidt reaction of the type would constitute an attractive entry into ring systems sporting a nitrogen atom at one of the ring fusion positions. In 1991, Aube and coworkers reported intramolecular reaction of alkyl azides with ketones can be accomplished in high yield under remarkably mild and straightforward reaction conditions.[b]
-
Literature reference
[a] Boyer, J. H.; Hamer, J. J. Am. Chem. Soc. 1955, 77 , 951. DOI:10.1021/ja01609a045
[b] Aube, J.; Milligan, G. L. J. Am. Chem. Soc. 1991, 113, 8965. DOI: 10.1021/ja00023a065
・Aube, J.; Milligan, G. L.; Mossman, C. J. J. Org. Chem. 1992, 57, 1635. DOI: 10.1021/jo00032a003
・Gracias, V.; Milligan, G. L.; Aube, J. J. Am. Chem. Soc. 1995, 117, 8047. DOI: 10.1021/ja00135a036
・Gracias, V.; Frank, K. E.; Milligan, G. L.; Aube, J. Tetrahedron 1997, 53, 16241. DOI:10.1016/S0040-4020(97)01012-0
・Desai, P.; Schildknegt, K.; Agrios, K. A.; Mossman, C.; Milligan, G. L.; Aube, J. J. Am. Chem. Soc.2000, 122, 7226. DOI: 10.1021/ja000490v
・Grecian,S.; Aube, J. Org. Synth. 2007, 84, 347. [website]
-
Reaction mechanism
There are a variety of mechanistic possibilities available to the reaction. Most similar to the classical Schmidt reaction mechanism would be the simple Lewis acid activation of the carbonyl followed by the addition of alkyl azide.
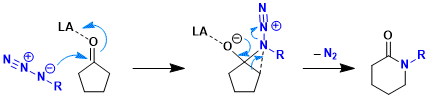
As noted above, similarities to the Schmidt reaction of hydrazoic acid with ketones must stop with the formation of the initial azidohydrin adduct as dehydration to afford an iminodiazonium ion is not an option for this intermediate. Once formed, it can only undergo bond reorganization to yield the product lactam or revert back to starting materials.
-
Example of reactions
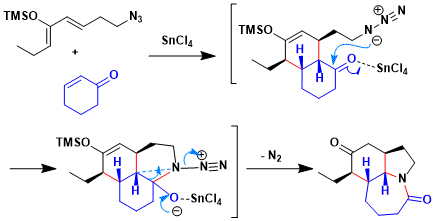
This work demonstrates the utility of the Diels-Alder/Schmidt reaction sequence for complex synthesis. Azide compounds was reacted cyclohexenone with BF3•OEt2, which afforded exclusively endo material in modest yield.
-
Bibliography
[1] (a) “An Expeditious Total Synthesis of (±)-Stenine”
Zeng, Y.; Aube, J. J. Am. Chem. Soc. 2005, 127, 15712. DOI: 10.1021/ja055629m
(±)-Stenine was synthesized in eight steps from a known ketophosphonate reagent. The key step was an exo-selective Diels−Alder/intramolecular Schmidt domino reaction that afforded three of the four rings and four stereocenters in a single reaction.
(b)”Syntheses of the Stemona Alkaloids (±)-Stenine, (±)-Neostenine, and (±)-13-Epineostenine Using a Stereodivergent Diels–Alder/Azido-Schmidt Reaction”
Frankowski, K. J.; Golden, J. E.; Zeng, Y.; Lei, Y.; Aube, J. J. Am. Chem. Soc. 2008, 130, 6018. DOI:10.1021/ja800574m
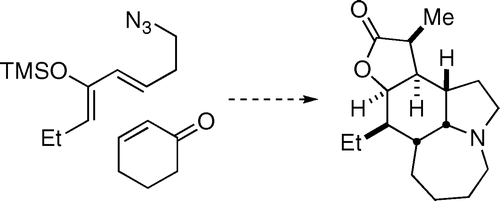
A tandem Diels–Alder/azido-Schmidt reaction sequence provides rapid access to the core skeleton shared by several Stemona alkaloids including stenine, neostenine, tuberostemonine, and neotuberostemonine. The discovery and evolution of inter- and intramolecular variations of this process and their applications to total syntheses of (±)-stenine and (±)-neostenine are described. The stereochemical outcome of the reaction depends on both substrate type and reaction conditions, enabling the preparation of both (±)-stenine and (±)-neostenine from the same diene/dienophile combination.
-
Related Books
[amazonjs asin=”1118356365″ locale=”US” title=”Organic Reactions (Volume 78)”]
-
Related Links
Schmidt Rearrangement - Wikipedia Jeff Aube