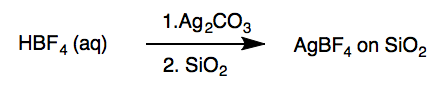- Popularity
- Responsibility
- Criteria #3
- Criteria #4
- Criteria #5
-
Characteristics
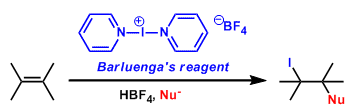
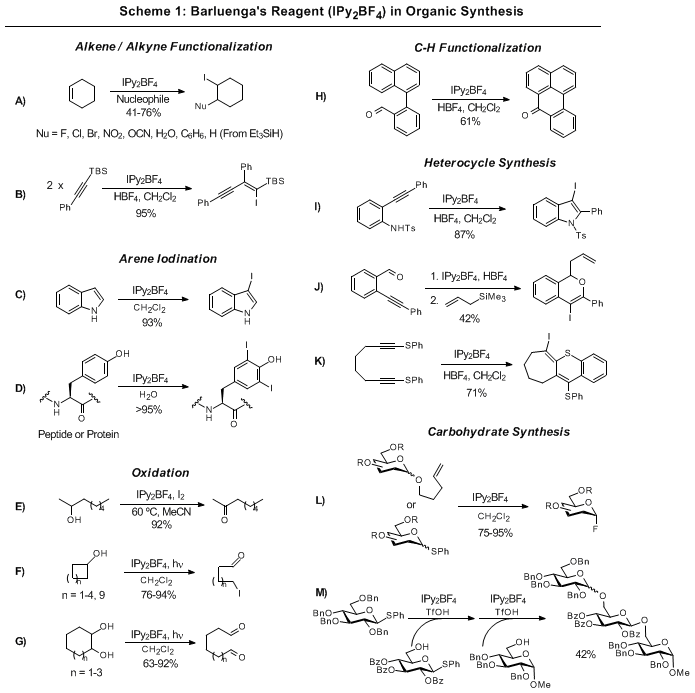
Bis(pyridine)iodonium tetrafluoroborate (IPy2BF4; CAS 15656-28-7) is an iodinating and oxidizing reagent[b], which was developed by José Barluenga.[a] Examples as an iodinating reagent include all kinds of unsaturated substrates such as isolated and conjugated olefins, acetylenes and aromatic compounds tolerating a variety of functional groups. The reagent is conveniently prepared by reaction of HgO/HBF4 -SiO2 or AgBF4-SiO2 with iodine and pyridine in CH2Cl2.[c]
-
Literature reference
[a] Barluenga, J. Pure. Appl. Chem. 1999, 71, 431. doi:10.1351/pac199971030431
[b] Barluenga, J.; Gonzalez-Bobes, F.; Murguia, M. C.; Ananthoju, S, R,; Gonzalez, J. M. Chem. Eur. J.2004, 10, 4206. DOI:10.1002/chem.200400136
[c] Chalker, J. M.; Thompson, A. L.; Davis, B. G. Org. Synth. 2010, 87, 288. [website]
-
Example of reactions
-
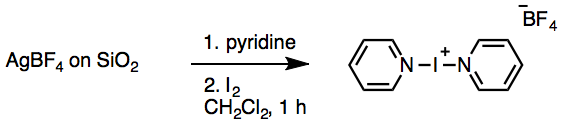
Preparation of Barluenga’s Reagent[c]
Silver tetrafluoroborate on SiO2. A 500-mL, one-necked round-bottomed flask is equipped with a Teflon-coated magnetic stir bar (3.9 cm × 1 cm, rectangular). Deionized water(50 mL) is poured into the flask and tetrafluoroboric acid (6.3 mL, 50 mmol, 1.0 equiv) is added by syringe. The solution is stirred, open to air, at room temperature (23 °C) for 2 min and silver carbonate (6.89 g, 25.0 mmol, 0.50 equiv) is added in several portions with a spatula over 2 min. Carbon dioxide gas evolves. The resulting mixture is stirred rapidly (380 rpm) until all solids are dissolved to give a grey solution (20 min). Silica gel (10.0 g) is poured into the solution and the mixture is stirred for 5 min. The stir bar is removed using a magnetic wand, rinsed with deionized water (1 mL) with a glass pipette, and the flask is transferred to a rotary evaporator into a water bath pre-heated to 55 °C. The water in the reaction flask is evaporated (55 °C, 80 mmHg, cooling is effected by circulating chilled water through the condenser) over 35 min to give the AgBF4 on SiO2 as an off-white/grey solid on the walls of the flask. A spatula is used to scrape all solids to the bottom of the flask, which is then returned to the rotary evaporator and dried with rapid spinning (55 °C, 80 mmHg). After 15 min of drying in this manner, a spatula is used to scrape all solids to the bottom of the flask. A free flowing powder is obtained. The flask is allowed to stand and cool at room temperature for 5 min before the mixture is used directly in the next step.
Bis(pyridine)iodonium(I) tetrafluoroborate (IPy2BF4). A Teflon-coated magnetic stir bar (3.9 cm × 1 cm, rectangular) is added to the 500-mL 1-necked flask that contains the AgBF4 on SiO2 from above. Dichloromethane (300 mL) is poured into the flask and the mixture is stirred at room temperature, open to the atmosphere. Pyridine (8.1 mL, 100 mmol, 2.0 equiv) is poured into the reaction flask from a 10.0 mL measuring cylinder, and the mixture is stirred at room temperature. One minute after the addition ofpyridine, iodine flakes (12.69 g, 50.0 mmol, 1.0 equiv) are poured into the reaction flask from a weighing paper. A yellow precipitate (AgI) is formed immediately upon addition of the iodine. A glass stopper is used to loosely cap the reaction flask and the mixture is stirred vigorously (380 rpm) for 1 h. The solution gradually turns red as the iodine is dissolved. After 1 h of stirring, the solids are removed by filtration through a sintered glass funnel under suction (15 mmHg), and the dark red filtrate is collected directly in a 1-L, 1-necked round-bottomed flask using a 24/40 glass filtration adapter. The reaction flask is rinsed with dichloromethane (100 mL) to ensure complete transfer, and the SiO2/AgI filter cake is then washed with additional dichloromethane (100 mL). The solvent is removed by rotary evaporation (35 °C, 80 mmHg) to give a reddish-yellow solid. This solid is then dissolved in dichloromethane (100 mL) and a Teflon-coated stir bar (5 cm × 1 cm, rectangular) is added. The solution is stirred in an ice bath for 5 min and then diethyl ether(200 mL) is poured into the stirred solution to precipitate the product. The mixture is stirred at 0 °C for 10 min to ensure that all IPy2BF4 is precipitated. The product is isolated by filtration under suction (15 mmHg). The stirrer is removed by a magnetic wand, held over the filter funnel and washed with diethyl ether (4) using a glass pipette. The flask is rinsed with diethyl ether (50 mL) to complete the transfer. The off-white powder is dried on the filter with suction for 5 min, transferred to a weighing paper (6″ × 6″) and dried under vacuum in a desiccator for 4 h (1.2 mmHg) to obtain 1 (13.67 g, 73%). This material is used for NMR, IR and Mp analysis. IPy2BF4 can also be recrystallized from dichloromethane. Accordingly, the powdered 1 (13.64 g) is added to a 100-mL, 1-necked round-bottomed flask with a Teflon-coated stir bar (1.5 cm × 1 cm, rectangular). A water bath is preheated to 50 °C and dichloromethane (25 mL) is added to the solid. The suspension is stirred (870 rpm) and lowered into the water bath. Once the dichloromethane is boiling (ca. 1 min), additional dichloromethane is added by glass pipette, with stirring, until all solids are dissolved (48 mL of additional dichloromethane, ca. 73 mL of total solvent). Once a clear solution is obtained, the stirring is stopped and the flask removed from the water bath. Crystals are observed within 3 min. After 30 min, the flask is cooled to 0 °C (ice bath) to facilitate the crystallization. After 2 h on ice, the crystals are isolated by filtration with suction (15 mmHg) , collecting the filtrate directly in a 250-mL round-bottomed flask. The suction is removed, the stir bar is held over the filter funnel with a magnetic wand and washed with diethyl ether (2 mL), and the crystals are triturated with diethyl ether (30 mL) on the filter. The crystals are then dried on the filter for 5 min with suction, transferred to a jar and dried under vacuum in a desiccator for 2 h (1.2 mmHg). The first crop of crystals yields 8.34 g. All remaining material in the crystallization flask and filter is collected by rinsing with dichloromethane (50 mL). The combined filtrates and mother liquor are concentrated by rotary evaporation (35 °C, 80 mm Hg) to give a reddish yellow solid. The recrystallization is repeated twice more to recover the remaining material, using dichloromethane (24 mL) in a 50-mL round-bottomed flask in the second recrystallization (2.46 g of crystals isolated) and dichloromethane (23 mL) in a 25-mL round-bottomed flask in the third and final recrystallization (0.79 g of crystals isolated). The total recovery of 1 from recrystallization is 11.59 g (85% from powdered IPy2BF4 and 62% overall).
-
Related Books
[amazonjs asin=”1118341031″ locale=”US” title=”Hypervalent Iodine Chemistry: Preparation, Structure, and Synthetic Applications of Polyvalent Iodine Compounds”][amazonjs asin=”3540441077″ locale=”US” title=”Hypervalent Iodine Chemistry: Modern Developments in Organic Synthesis (Topics in Current Chemistry)”]
-
Related Links
José Barluenga: Baran Lab meeting
José Barluenga: Angewandte Author's profile