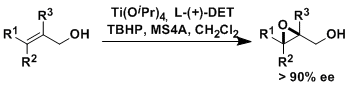Wang, C.; Yamamoto, H. J. Am. Chem. Soc. 2014, ASAP.
DOI: 10.1021/ja411379e
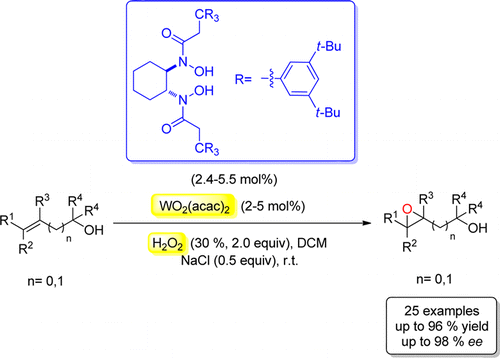
A simple, efficient, and environmentally friendly asymmetric epoxidation of primary, secondary, tertiary allylic, and homoallylic alcohols has been accomplished. This process was promoted by a tungsten–bishydroxamic acid complex at room temperature with the use of aqueous 30% H2O2 as oxidant, yielding the products in 84–98% ee.
Asymmetric epoxidation (AE), represented as Sharpless-Katsuki AE, is one of the most important transformations in organic synthesis. Therefore, several methods of AE have been developed so far , for examples, Shi AE and Jacobsen-Katsuki AE.
Sharpless-Katsuki AE
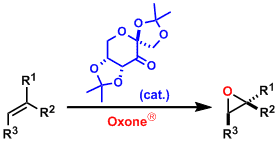
Shi AE
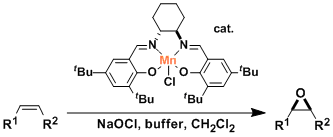
Jacobsen-Katsuki AE
In recent years, Prof. Hisashi Yamamoto group developed a series of unique bishydroxamic acids (BHA) and applied them successfully as ligands for highly enantioselective epoxidation of allylic, homoallylic, and bishomoallylic alcohols as well as N-alkenyl sulfonamides and N -tosyl imines.[1] However, all these reactions employ toxic alkyl peroxide as the oxidant, functioning with low atom economy. This time, they report a tungsten-catalyzed asymmetric epoxidation of both primary, secondary, and tertiary allylic as well as homoallylic alcohols with aqueous H2O2 as oxidant by using our W-BHA catalyst system.
-
References
[1] “Hydroxamic Acids in Asymmetric Synthesis”
Li, Z.; Yamamoto, H. Acc. Chem. Res 2013, 46, 506–518. DOI:10.1021/ar300216r
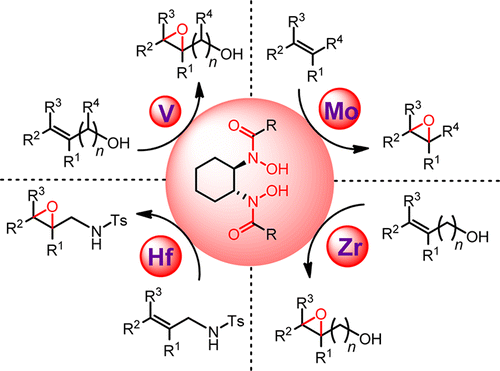
Metal-catalyzed stereoselective reactions are a central theme in organic chemistry research. In these reactions, the stereoselection is achieved predominantly by introducing chiral ligands at the metal catalyst’s center. For decades, researchers have sought better chiral ligands for asymmetric catalysis and have made great progress. Nevertheless, to achieve optimal stereoselectivity and to catalyze new reactions, new chiral ligands are needed.
Because of their high metal affinity, hydroxamic acids play major roles across a broad spectrum of fields from biochemistry to metal extraction. Dr. K. Barry Sharpless first revealed their potential as chiral ligands for asymmetric synthesis in 1977: He published the chiral vanadium-hydroxamic-acid-catalyzed, enantioselective epoxidation of allylic alcohols before his discovery of Sharpless asymmetric epoxidation, which uses the titanium–tartrate complex as the chiral reagent. However, researchers have reported few highly enantioselective reactions using metal-hydroxamic acid as catalysts since then.
This Account summarizes our research on metal-catalyzed asymmetric epoxidation using hydroxamic acids as chiral ligands. We designed and synthesized a series of new hydroxamic acids, most notably the C2-symmetric bis-hydroxamic acid (BHA) family. V-BHA-catalyzed epoxidation of allylic and homoallylic alcohols achieved higher activity and stereoselectivity than Sharpless asymmetric epoxidation in many cases. Changing the metal species led to a series of unprecedented asymmetric epoxidation reactions, such as (i) single olefins and sulfides with Mo-BHA, (ii) homoallylic and bishomoallylic alcohols with Zr- and Hf-BHA, and (iii) N-alkenyl sulfonamides and N-sulfonyl imines with Hf-BHA. These reactions produce uniquely functionalized chiral epoxides with good yields and enantioselectivities.
[amazonjs asin=”3527312137″ locale=”US” title=”Aziridines and Epoxides in Organic Synthesis”][amazonjs asin=”1243730005″ locale=”US” title=”Asymmetric epoxidation of various olefins catalyzed by fructose- and glucose-derived ketones.”]