- Importance as Chemical Concept
- Criteria #2
- Criteria #3
- Criteria #4
- Criteria #5
-
General Characteristics
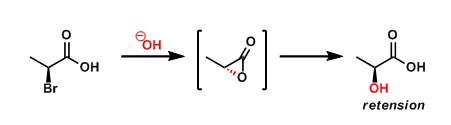
When the functional group(s) located near the reaction center directly influences the reactivity or selectivity of a given reaction, the effect is referred to as neighboring group participation.
For example, in the scheme shown above, the neighboring carboxylic acid participates in the reaction as an intramolecular nucleophile to effect the SN2 inversion, and the subsequent intermolecular SN2 inversion results in net retention of stereochemistry.
-
Reaction Mechanism
In many cases of neighboring group participation, the intramolecular process is the rate-determining step.
-
Examples
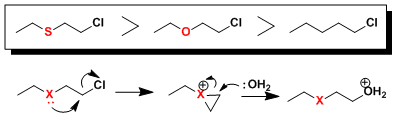
The relative rate of hydrolysis for selected alkyl chlorides is as follows. This can be rationalized if one considers three-membered “-onium” species as the intermediate.
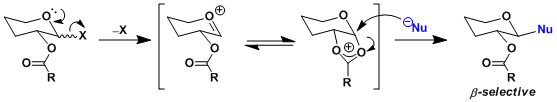
When the 2-hydroxyl group of a sugar is acylated, the glycosylation reaction is known to occur with high β-selectivity. This observation is generally explained by the neighboring group participation of the acyl group interacting with the oxonium intermediate.
-
External Links
Neighboring Group Participation – Wikipedia