- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
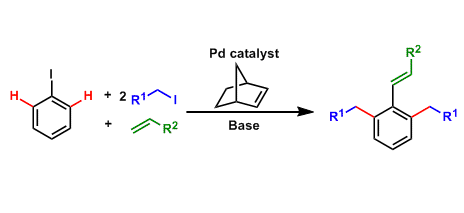
The Catellani reaction is a type of three component reaction in which the Mizoroki-Heck reaction and ortho C-H alkylation with alkyl halide occur concomitantly in the presence of norbornene as a cocatalyst with palladium.
-
General References
- Catellani, M.; Frignani, F.; Rangoni, A. Angew. Chem. Int. Ed. Engl. 1997, 36, 119. DOI: 10.1002/anie.199701191
- Catellani, M.; Cugini, F. Tetrahedron 1999, 55, 6595. doi:10.1016/S0040-4020(99)00293-8
- Catellani, M.; Motti, E.; Minari, M. Chem. Commun. 2000, 157. DOI: 10.1039/A909099A
- Catellani, M. Synlett 2003, 298. DOI: 10.1055/s-2003-37102
- Motti, E.; Ippomei, G.; Deledda, S.; Catellani, M. Synthesis 2003, 2671. DOI: 10.1055/s-2003-42441
- Faccini, F.; Motti, E.; Catellani, M. J. Am. Chem. Soc. 2004, 126, 78. DOI: 10.1021/ja039043g
- Ferraccioli, R.; Carenzi, D.; Catellani, M. Tetrahedron Lett. 2004, 45, 6903. doi:10.1016/j.tetlet.2004.07.090
- Catellani, M. Top. Organomet. Chem. 2005,14, 21.
- Catellani, M.; Motti, E Della Ca, N. Acc. Chem. Res. 2008, 41, 1512. DOI: 10.1021/ar800040u
- Ferraccioli, R. Synthesis 2013, 45, 581. DOI: 10.1055/s-0032-1318218
-
History
The Catellani reaction was developed and reported by Professor Marta Catellani of University of Parma in 1997.
-
Reaction Mechanism
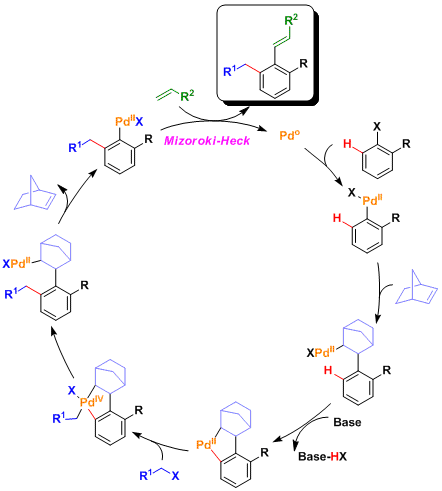
The catalytic cycle is thought to involve Pd(0), (II), and (IV) species. The migratory insertion into norbornene (which is reactive due to ring strain) leads to the formation of the intermediate that undergoes proximity-driven ortho C-H activation. (Ref: J.Organomet. Chem. 1993,458, C12 , Synthesis1996, 769)
Computational analysis of reaction mechanism: (Catellani M. et al, J. Am. Chem. Soc. 2011, 133, 8574. DOI: 10.1021/ja110988p )
-
Examples
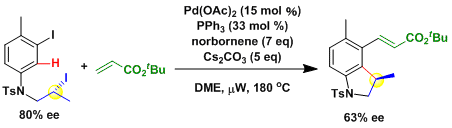
The oxidative addition to the alkyl halide proceeds with stereoinversion.[1]
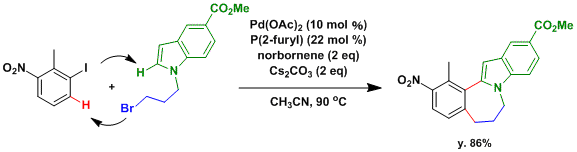
The Catellani reaction is particularly useful in the synthesis of polycyclic and heterocyclic compounds, such as the example shown here.[2]
-
References
[1] Rudolph, A.; Rackelmann, N.; Lautens, M. Angew. Chem. Int. Ed. 2007, 46, 1485. DOI: 10.1002/anie.200603888 [2] Bressy, C.; Alberico, D.; Lautens, M. J. Am. Chem. Soc. 2005, 127, 13148. DOI: 10.1021/ja054472v
-
Related Books
[amazonjs asin=”3642123554″ locale=”US” title=”C-H Activation (Topics in Current Chemistry)”]
-
External Links