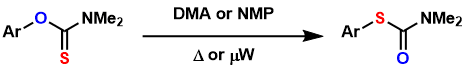- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
The thermal rearrangement of O-thiocarbamates into S-thiocarbamates is an effective way to synthesize thiophenols from phenols.
The rearrangement usually requires very high thermal conditions (200~300 °C) to proceed, thus microwave irradiation is helpful. Recently, palladium catalysis and photocatalysis have been shown to facilitate the reaction under milder conditions.
-
General References
- Newman, M. S.; Karnes, H. A. J. Org. Chem. 1966,31, 3980. doi:10.1021/jo01350a023
- Kwart, H.; Evans, E. R. J. Org. Chem. 1966, 31, 410. doi:10.1021/jo01340a015
- Moseley, J. D.; Sankey, R. F.; Tang, O. N.; Gilday, J. P. Tetrahedron, 2006, 62, 4685. doi:10.1016/j.tet.2005.12.063
- Moseley, J. D.; Lenden, P. Tetrahedron, 2007, 63, 4120. doi:10.1016/j.tet.2005.12.063
- Lloyd-Jones, G, C,; Moseley, J. D.; Rennya, J. S. Synthesis 2008, 661. DOI: 10.1055/s-2008-1032179
- Burns, M.; Lloyd-Jones, G. C.; Moseley, J. D.; Renny, J. S. J. Org. Chem. 2010, 75, 6347. DOI: 10.1021/jo1014382
-
Reaction Mechanism
The O to S rearrangement is driven by the conversion of C=S bond into thermodynamically more stable C=O bond. Since the reaction involves aromatic nucleophilic attack by the sulfur atom, the substrates containing electron-withdrawing groups at ortho or para position react faster.

The nitrogen must be dialkylated, as protonated O-thiocarbamates are prone to decomposition into isothiocyanates via the following pathway.

-
Examples
The reaction can be run at lower temperature (~100 °C) in the presence of a palladium catalyst.[1]

Ambient-temperature Newman-Kwart rearrangement mediated by organic photoredox catalysis[2]: This recently developed procedure allows the reaction to proceed at room temperature and tolerates a wide range of functional groups.

-
References
- Harvey, J. N.; Jover, J.; Lloyd-Jones, G. C.; Moseley, J. D.; Murray, P.; Renny, J. S. Angew. Chem. Int. Ed., 2009, 48, 7612. DOI: 10.1002/anie.200903908
- Perkowski, A. J.; Cruz, C. L.; Nicewicz, D. A. J. Am. Chem. Soc. 2015, 137, 15684. DOI: 10.1021/jacs.5b11800
-
External Links
- Newman-Kwart Rearrangement (Organic Chemistry Portal)
- Newman-Kwart Rearrangement – Wikipedia