- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
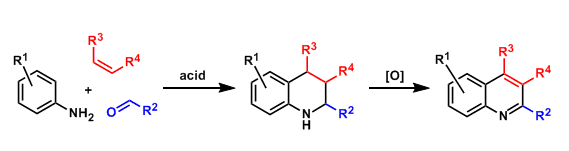
The condensation of aldehydes, anilines, and electron-rich alkenes provides access to substituted tetrahydroquinoline skeletons. This is a type of three-component reaction involving hetero Diels-Alder cycloaddition. Tetrahydroquinolines can be oxidized to quinolines if needed.
-
General References
- Povarov, L. S.; Mikhailov, B. M. Izv. Akad. Nauk SSR, Ser. Khim. 1963, 953.
- Povarov, L. S.; Grigos, V. I.; Mikhailov, B. M. Izv. Akad. Nauk SSR, Ser. Khim. 1963, 2039.
- Povarov, L. S. Russ. Chem. Rev. 1967, 36, 656.
- Buonora, P.; Olsenb, J.-C.; Oh, T. Tetrahedron 2001, 57, 6099. doi:10.1016/S0040-4020(01)00438-0
- Kouznetsov, V. V. Tetrahedron 2009, 65, 2721. doi:10.1016/j.tet.2008.12.059
- Bello, D.; Ramon, R.; Lavilla, R. Curr. Org. Chem. 2010, 14, 332.
-
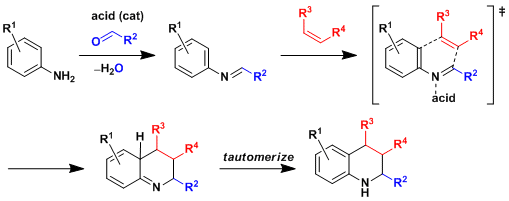
Reaction Mechanism
-
Examples
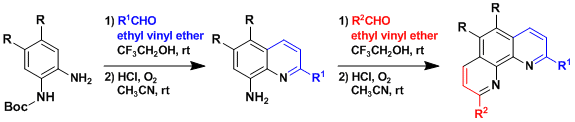
The synthesis of unsymmetrical phenanthrolines.[1]
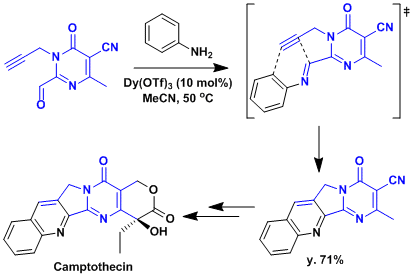
The application to the synthesis of camptothecin.[2]
-
Experimental Tips
-
References
[1] De, K.; Legros, J.; Crousse, B.; Chandrasekaran, S.; Bonnet-Delpon, D. Org. Biomol. Chem. 2011, 9, 347. DOI: 10.1039/C0OB00496K
[2] Twin, H.; Batey, R. A. Org. Lett. 2004, 6, 4913. DOI: 10.1021/ol0479848
-
Related Reactions
-
Related Books
[amazonjs asin=”0121108600″ locale=”US” title=”Hetero Diels-Alder Methodology in Organic Synthesis (Organic Chemistry)”]
[amazonjs asin=”3527301593″ locale=”US” title=”Cycloaddition Reactions in Organic Synthesis”]
-
External Links