- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
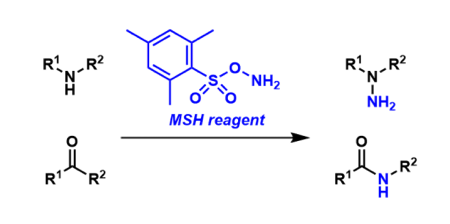
MSH (O-(mesitylsulfonyl)hydroxylamine) is an electrophilic aminating agent used to introduce a NH2 unit. It is used in reactions such as the Beckmann rearrangement, the Neber rearrangement, and N-amination reactions. It is not commercially available becuase it can detonate when it is concentrated to dryness. It has to be used with appropriate care when it needs to be used.
A similar reagent to MSH is O-(2,4-dinitrophenyl)hydroxylamine, which is relatively easy to handle and can be prepared on larger scales.

-
General References
<reviews for MSH>
- Tamura, Y.; Minamikawa, J.: Ikeda, M. Synthesis 1977, 1. DOI: 10.1055/s-1977-24260
- Erdik, E.; Ay, M. Chem. Rev. 1989, 89, 1947. DOI: 10.1021/cr00098a014
<O-(2,4-dinitrophenyl)hydroxylamine>
- Legault, C.; Charette, A. B. J. Org. Chem. 2003, 68, 7119. DOI: 10.1021/jo034456l
- Yang, Z. Synlett 2014, 25, 1186. DOI: 10.1055/s-0033-1341110
-
Reaction Mechanism
-
Examples
The total synthesis of pinnaic acid with the MSH-mediated Beckmann rearrangement as the key step.[1] 
The conversion of boronic acids to anilines.[2] 
The application to aziridine synthesis.[3] 
-
Experimental Procedure
The preparation of O-(2,4-dinitrophenyl)hydroxylamine.[4] 
-
Experimental Tips
-
References
- Xu, S.; Arimoto, H.; Uemura, D. Angew. Chem. Int. Ed. 2007, 46, 5764. DOI:10.1002/anie.200701581
- Zhu, C.; Li, G.; Ess, D. H.; Falck, J. R.; Kurti, L. J. Am. Chem. Soc. 2012, 134, 18253. DOI: 10.1021/ja309637r
- Jat, J. L.; Paudyal, M. P.; Gao, H.; Xu, Q.-L.; Yousufuddin, M.; Devarajan, D.; Ess, D. H.; Kurti, L.; Falck, J. R. Science 2014, 343, 61. DOI:10.1126/science.1245727
- Legault, C.; Charette, A. B. J. Org. Chem. 2003, 68, 7119. DOI: 10.1021/jo034456l
-
Related Reactions
-
Related Books
-
External Links