- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
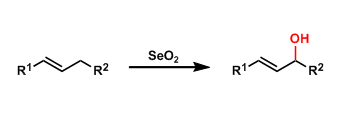
Selenium dioxide oxidizes allylic C-H bonds, providing an option to synthesize allylic alcohols from alkenes. Even though SeO2 is a toxic reagent, it is used frequently since it mediates a transformation that is hard to effect by other means. The reaction conditions are mild too.
When re-oxidants such as TBHP (tert-butyl hydroperoxide) are used, SeO2 can be reduced to substoichiometric amounts.
-
General References
- Riley, H. L.; Morley, J. F.; Friend, N. A .C. J. Chem. Soc. 1932, 1875.
- Rabjohn, N. Org. React. 1949, 5, 331.
- Rabjohn, N. Org. React. 1976, 24, 261.
- Bulman Page, P. C.; McCarthy, T. J. Comprehensive Organic Synthesis 1991,7, 84, 108.
-
Reaction Mechanism
The reaction mechanism is generally explained by the ene reaction followed by [2,3]-sigmatropic rearrangement. (Ref: J. Org. Chem. 2000, 65, 7554., Tetrahedron Lett. 2003, 44, 1099.)
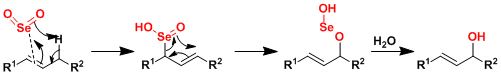
-
Examples
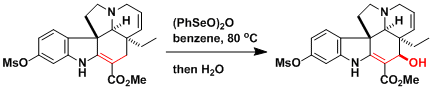
The total synthesis of vinblastine.[1]
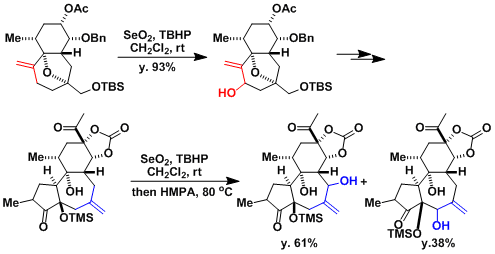
The total synthesis of resiniferatoxin.[2]
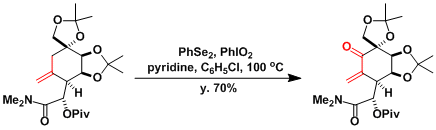
The total synthesis of tetrodotoxin[3]: Under the conditions using PhSeSePh/PhIO2/Py (the Barton modification),[4] the substrates are oxidized all the way to enones.
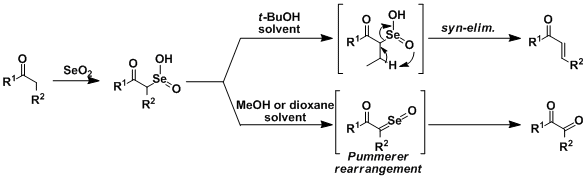
The α-position of carbonyl compounds is also susceptible to oxidation by SeO2. Various oxidation products can be synthesized depending on the conditions.
-
Experimental Procedure
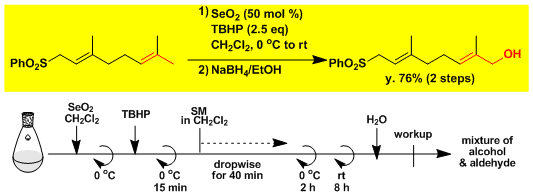
Allylic oxidation of geranyl sulfone.[5]
-
Experimental Tips
-
References
[1] Fukuyama,T. et al. J. Am. Chem. Soc. 2002, 124, 2137. DOI: 10.1021/ja0177049 [2] Wender, P. A. et al. J. Am. Chem. Soc. 1997, 119, 12976. DOI: 10.1021/ja972279y [3] Hinman, A.; Du Bois, J. J. Am. Chem. Soc. 2003, 125, 11510. DOI: 10.1021/ja0368305 [4] Barton, D. H. R.; Crich, D. Tetrahedron 1985, 41, 4395. doi:10.1016/S0040-4020(01)97207-2 [5] Marshall, J. A.; Andrews, R. C. J. Org. Chem. 1985, 50, 1602. DOI: 10.1021/jo00210a009
-
Related Reactions
- Kharasch-Sosnovsky Oxidation
- Cross Dehydrogenative Coupling (CDC)
- Catalytic C-H Oxidation
- Hofmann-Löffler-Freytag Reaction
- Meisenheimer Rearrangement
- Catalytic C-H activation
- C-H Insertion of Metal Carbenoid
- Mislow-Evans Rearrangement
-
Related Books
-
External Links
- Selenium Dioxide (Wikipedia)
- Reley Oxidation – Wikipedia