- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
Upon treatment with strong bases (e.g. in aqueous NaOH) and heating, non-enolizable aldehydes undergo redox disproportionation to give corresponding alcohols and carboxylic acids in 1:1 ratio.
The crossed Cannizzaro reaction between two different aldehydes is difficult and a statistical mixture of four products is usually expected. However, when one of the aldehydes is formaldehyde, it reacts as a reducing agent more preferentially, giving the alcohol derived from the other aldehyde in good yield.
The Cannizzaro reaction doesn’t work well for enolizable aldehydes due to competing aldol reaction.
-
General References
・Cannizzaro, S. Ann. 1853, 88, 129. doi:10.1002/jlac.18530880114
・List, K.; Limpricht, H. Ann, 1854, 90, 190. doi:10.1002/jlac.18540900211
・Geissman, T. A. Org. React. 1944, 2, 94.
-
Reaction Mechanism
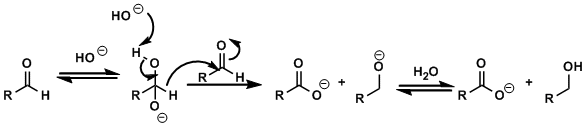
-
Examples
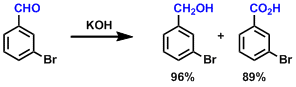
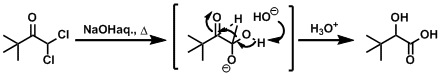
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Books

