- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
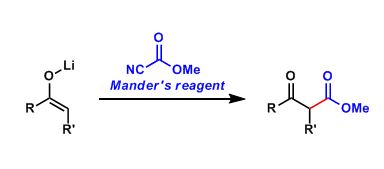
Methyl cyanoformate, also known as Mander’s reagent, is used to introduce a methoxycarbonyl group to metal enolates for the synthesis of β-ketoesters.
Compared with chloroformate esters, which tend to react with enolates to give a mixture of C– and O-carboxylated products, Mander’s reagent is more C-selective.
It serves as a source of cyanide as well, such as for the addition of cyanide to carbonyls in the presence of nucleophilic or Lewis acidic catalysts.
-
General References
- Mander, L. N.; Sethi, S. P. Tetrahedron Lett. 1983, 24, 5425. doi:10.1016/S0040-4039(00)87886-7
- Crabtree, S. R.; Chu, W. L. A.; Mander, L. N. Synlett 1990, 169. DOI: 10.1055/s-1990-21025
- Bissember, A. C. Synlett 2009, 681. DOI: 10.1055/s-0028-1087716
-
Reaction Mechanism
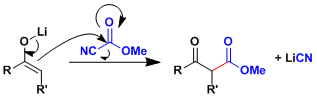
-
Examples
An appearance in the synthesis of strychnine.[1]

An application to the synthesis of ingenol.[2]

-
Experimental Tips
-
References
[1] Knight, S. D.; Overman, L. E.; Pairaudeau, G. J. Am. Chem. Soc. 1993, 115, 9293. doi:10.1021/ja00073a057
[2] Winkler, J. D.; Rouse, M. B.; Greaney, M. F.; Harison, S. J.; Jeon, Y. T. J. Am. Chem. Soc. 2002, 124, 9726. DOI: 10.1021/ja026600a
-
Related Reactions
-
Related Books
-
External Links
- Methyl cyanoformate – Wikipedia
- The Chemistry of Lewis N. Mander (PDF, Baran’s group seminar)